Abstract
Machines at a molecular level are in perpetual Brownian motion even at an ambient temperature. One of the representative issues of researches on molecular machines is a development of technology, which can control Brownian motion. This review presents our efforts to achieve the first rationally designed molecular brake systems of threading/dethreading motions, a shuttling motion, and a rocking motion that work reversibly and quantitatively in response to external stimuli without producing any chemical wastes. These molecular brake systems were constructed from a dumbbell shaped secondary ammonium axle and a ring component having photo and thermally reactive moiety.





























Similar content being viewed by others
References
Pedersen, C.J.: Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 89, 2495–2496 (1967)
Pedersen, C.J.: Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 89, 7017–7036 (1967)
Pedersen, C.J.: The discovery of crown ethers (noble lecture). Angew. Chem. Int. Ed. Engl. 27, 1021–1027 (1988)
Pedersen, C.J.: The discovery of crown ethers. J. Incl. Phenom. 6, 337–350 (1988)
Cram, D.J.: The design of molecular hosts, guests, and their complexes (nobel lecture). Angew. Chem. Int. Ed. Engl. 27, 1009–1020 (1988)
Cram, D.J.: The design of molecular hosts, guests, and their complexes. J. Incl. Phenom. 6, 397–413 (1988)
Lehn, J.M.: Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices. Angew. Chem. Int. Ed. Engl. 27, 89–112 (1988)
Lehn, J.M.: Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices. J. Incl. Phenom. 6, 351–396 (1988)
Lehn, J.M.: Supramolecular Chemistry—Concepts and Perspectives. VCH, Weinheim (1995)
Izatt, R.M., Bradshaw, J.S., Nielsen, S.A., Lamb, J.D., Christensen, J.J.: Thermodynamic and kinetic data for cation macrocycle interaction. Chem. Rev. 85, 271–339 (1985)
Izatt, R.M., Pawlak, K., Bradshaw, J.S., Bruening, R.L.: Thermodynamic and kinetic data for macrocycle interaction with cations and anions. Chem. Rev. 91, 1721–2085 (1991)
Izatt, R.M., Pawlak, K., Bradshaw, J.S., Bruening, R.L.: Thermodynamic and kinetic data for macrocycle interaction with cations, anions, and neutral molecules. Chem. Rev. 95, 2529–2586 (1995)
Izatt, R.M., Bradshaw, J.S., Pawlak, K., Bruening, R.L., Tarbet, B.J.: Thermodynamic and kinetic data for macrocycle interaction with neutral molecules. Chem. Rev. 92, 1261–1354 (1992)
Zhang, X.X., Bradshaw, J.S., Izatt, R.M.: Enantiomeric recognition of amine compounds by chiral macrocyclic receptors. Chem. Rev. 97, 3313–3361 (1997)
Kaneda, T., Hirose, K., Misumi, S.: Chiral azophenolic acerands: color indicator to judge the absolute configuration of chiral amines. J. Am. Chem. Soc. 111, 742–743 (1989)
Naemura, K., Mizo-Oku, T., Kamada, K., Hirose, K., Tobe, Y., Sawada, M., Takai, Y.: Preparation of homochiral crown ether containing (S)-1-(1-adamantyl)ethane-1, 2-diol as a chiral subunit and its enantioselective complexation with an organic ammonium cation. Tetrahedron Asymmetr. 5, 1549–1558 (1994)
Naemura, K., Asada, M., Hirose, K., Tobe, Y.: Preparation and enantiomer recognition of chiral azophenolic crown ethers having three chiral barriers on each of the homotopic faces. Tetrahedron Asymmetr. 6, 1873–1876 (1995)
Naemura, K., Takeuchi, S., Sawada, M., Ueno, K., Hirose, K., Tobe, Y., Kaneda, T., Sakata, Y.: Synthesis of azophenolic crown ethers of CS symmetry incorporating cis-1-phenylcyclohexane-1, 2-diol residues as a steric barrier and diastereotopic face selectivity in complexation of amines by their diastereotopic faces. J. Chem. Soc. Perkin Trans. 1, 1429–1435 (1995)
Naemura, K., Takeuchi, S., Hirose, K., Tobe, Y., Kaneda, T., Sakata, Y.: Preparation and enantiomer recognition behaviour of azophenolic crown ethers containing cis-cyclohexane-1, 2-diol as the chiral centre. J. Chem. Soc. Perkin Trans. 1, 213–219 (1995)
Naemura, K., Fuji, J., Ogasahara, K., Hirose, K., Tobe, Y.: Temperature dependent reversal of enantiomer selectivity in the complexation of optically active phenolic crown ethers with chiral amines. Chem. Commun., 2749–2750 (1996)
Naemura, K., Ueno, K., Takeuchi, S., Hirose, K., Tobe, Y., Kaneda, T., Sakata, Y.: Preparation and enantiomer recognition behaviour of azophenolic crown ethers containing cis-cyclohexane-1,2-diol as the chiral subunit and 2,4-dinitrophenylazophenol as the chromophore. J. Chem. Soc. Perkin Trans. 1, 383–388 (1996)
Hirose, K., Fuji, J., Kamada, K., Tobe, Y., Naemura, K.: Temperature dependent inversion of enantiomer selectivity in the complexation of optically active azophenolic crown ethers containing alkyl substituents as chiral barriers with chiral amines. J. Chem. Soc. Perkin Trans. 2, 1649–1657 (1997)
Naemura, K., Nishikawa, Y., Fuji, J., Hirose, K., Tobe, Y.: Preparation of homochiral phenolic crown ethers containing para-substituted phenol moiety and chiral subunits derived from (S)-1-phenylethane-1, 2-diol: Their chiral recognition behaviour in complexation with neutral amines. Tetrahedron Asymmetr. 8, 873–882 (1997)
Naemura, K., Ogasahara, K., Hirose, K., Tobe, Y.: Preparation of homochiral azophenolic crown ethers containing 1-phenylethane-1,2-diol and 2,4-dimethyl-3-oxapentane-1,5-diol as a chiral subunits: enantiomer recognition behaviour towards chiral 2-aminoethanol derivatives. Tetrahedron Asymmetr. 8, 19–22 (1997)
Naemura, K., Wakebe, T., Hirose, K., Tobe, Y.: Preparation of homochiral phenolic crown ethers having chiral subunits derived from (1R,2S)-cis-1,2,3,4-tetrahydronaphthalene-1,2-diol: temperature-dependent enantiomer selectivity in complexation with neutral amines. Tetrahedron Asymmetr. 8, 2585–2595 (1997)
Ogasahara, K., Hirose, K., Tobe, Y., Naemura, K.: Preparation of optically active azophenolic crown ethers containing 1-phenylethane-1,2-diol and 2,4-dimethyl-3-oxapentane-1,5-diol as a chiral subunits: temperature-dependent enantiomer selectivity in complexation with chiral amines. J. Chem. Soc. Perkin Trans. 1, 3227–3236 (1997)
Naemura, K., Matsunaga, K., Fuji, J., Ogasahara, K., Nishikawa, Y., Hirose, K., Tobe, Y.: Temperature dependence of enantiomer selectivity in complexations of optically active phenolic crown ethers with chiral amines in solution. Anal. Sci. 14, 175–182 (1998)
Naemura, K., Nishioka, K., Ogasahara, K., Nishikawa, Y., Hirose, K., Tobe, Y.: Preparation and temperature-dependent enantioselectivities of homochiral phenolic crown ethers having aryl chiral barriers: thermodynamic parameters for enantioselective complexation with chiral amines. Tetrahedron Asymmetr. 9, 563–574 (1998)
Hirose, K., Ogasahara, K., Nishioka, K., Tobe, Y., Naemura, K.: Enantioselective complexation of phenolic crown ethers with chiral aminoethanol derivatives: effects of substituents of aromatic rings of hosts and guests on complexation. J. Chem. Soc. Perkin Trans. 2, 1984–1993 (2000)
Hirose, K., Fujiwara, A., Matsunaga, K., Aoki, N., Tobe, Y.: Chiral recognition of secondary amines by using chiral crown ether and podand. Tetrahedron Lett. 43, 8539–8542 (2002)
Hirose, K., Fujiwara, A., Matsunaga, K., Aoki, N., Tobe, Y.: Preparation of phenolic chiral crown ethers and podands and their enantiomer recognition ability toward secondary amines. Tetrahedron Asymmetr. 14, 555–566 (2003)
Wenzel, T.J., Freeman, B.E., Sek, D.C., Zopf, J.J., Nakamura, T., Jin, Y.Z., Hirose, K., Tobe, Y.: Chiral recognition in NMR spectroscopy using crown ethers and their ytterbium(iii) complexes. Anal. Bioanal. Chem. 378, 1536–1547 (2004)
Hirose, K., Aksharanandana, P., Suzuki, M., Wada, K., Naemura, K., Tobe, Y.: Remarkable effect of subtle structural change of chiral pseudo-18-crown-6 on enantiomer-selectivity in complexation with chiral amino alcohols. Heterocycles 66, 405–431 (2005)
Hirose, K., Goshima, Y., Wakebe, T., Tobe, Y., Naemura, K.: Supramolecular method for the determination of absolute configuration of chiral compounds: theoretical derivatization and a demonstration for phenolic crown ether—2-amino-1-ethanol system. Anal. Chem. 79, 6295–6302 (2007)
Sawada, M., Okumura, Y., Shizuma, M., Takai, Y., Hidaka, Y., Yamada, H., Tanaka, T., Kaneda, T., Hirose, K., Misumi, S., Takahashi, S.: Enantioselective complexation of carbohydrate or crown ether hosts with organic ammonium ion guests detected by FAB mass spectrometry. J. Am. Chem. Soc. 115, 7381–7388 (1993)
Sawada, M., Okumura, Y., Yamada, H., Takai, Y., Takahashi, S., Kaneda, T., Hirose, K., Misumi, S.: Cross-chiral examinations of molecular enantioselective recognition by fast atom bombardment mass spectrometry: host–guest complexations between chiral crown ethers and chiral organic ammonium ions. Org. Mass Spectrom. 28, 1525–1528 (1993)
Sawada, M., Takai, Y., Yamada, H., Kaneda, T., Kamada, K., Mizooku, T., Hirose, K., Tobe, Y., Naemura, K.: Chiral recognition in molecular complexation for the crown ether-amino ester system. A facile FAB mass spectrometric approach. J. Chem. Soc. Chem. Commun., 2497–2498 (1994)
Sawada, M., Takai, Y., Yamada, H., Hirayama, S., Kaneda, T., Tanaka, T., Kamada, K., Mizooku, T., Takeuchi, S., Ueno, K., Hirose, K., Tobe, Y., Naemura, K.: Chiral recognition in host–guest complexation determined by the enantiomer-labeled guest method using fast atom bombardment mass spectrometry. J. Am. Chem. Soc. 117, 7726–7736 (1995)
Sawada, M., Takai, Y., Kaneda, T., Arakawa, R., Okamoto, M., Doe, H., Matsuo, T., Naemura, K., Hirose, K., Tobe, Y.: Chiral molecular recognition in electrospray ionization mass spectrometry. Chem. Commun., 1735–1736 (1996)
Sawada, M., Takai, Y., Yamada, H., Nishida, J., Kaneda, T., Arakawa, R., Okamoto, M., Hirose, K., Tanaka, T., Naemura, K.: Chiral amino acid recognition detected by electrospray ionization (ESI) and fast atom bombardment (FAB) mass spectrometry (MS) coupled with the enantiomer labelled (EL) guest method. J. Chem. Soc. Perkin Trans. 2, 701–710 (1998)
Sawada, M., Hagita, K., Imamura, H., Tabuchi, H., Yodoya, S., Umeda, M., Takai, Y., Yamada, H., Yamaoka, H., Hirose, K., Tobe, Y., Tanaka, T., Takahashi, S.: Chiral recognition ability of crown ethers toward organic amine compounds: FAB mass spectrometry coupled with the enantiomer-labeled guest method. J. Mass Spectrom. Soc. Jpn. 48, 323–332 (2000)
Sawada, M., Takai, Y., Imamura, H., Yamada, H., Takahashi, S., Yamaoka, H., Hirose, K., Tobe, Y., Tanaka, J.: Chiral recognizable host–guest interactions detected by fast-atom bombardment mass spectrometry: application to the enantiomeric excess determination of primary amines. Eur. J. Mass Spectrom. 7, 447–459 (2001)
Sawada, M., Takai, Y., Yamada, H., Yoshikawa, M., Arakawa, R., Tabuchi, H., Takada, M., Tanaka, J., Shizuma, M., Yamaoka, H., Hirose, K., Fukuda, K., Tobe, Y.: Depression of the apparent chiral recognition ability obtained in the host–guest complexation systems by electrospray and nano-electrospray ionization mass spectrometry. Eur. J. Mass Spectrom. 10, 27–37 (2004)
Nishioka, R., Ueshige, T., Nakamura, T., Hirose, K., Tobe, Y.: Preparation and evaluation of novel chiral stationary phases chemically bonded with chiral pseudo crown ether. Chromatography 21, 294–295 (2000)
Ueshige, T., Nishioka, R., Nakamura, T., Hirose, K., Tobe, Y.: Enantiomeric separations of stimulant materials using chiral stationary phase bonded with pseudo crown ether. Chromatography 21, 368–369 (2000)
Hirose, K., Nakamura, T., Nishioka, R., Ueshige, T., Tobe, Y.: Preparation and evaluation of novel chiral stationary phases covalently bound with chiral pseudo-18-crown-6 ethers. Tetrahedron Lett. 44, 1549–1551 (2003)
Hirose, K., Jin, Y.Z., Nakamura, T., Nishioka, R., Ueshige, T., Tobe, Y.: Chiral stationary phase covalently bound with a chiral pseudo-18-crown-6 ether for enantiomer separation of amino compounds using a normal mobile phase. Chirality 17, 142–148 (2005)
Hirose, K., Jin, Y.Z., Nakamura, T., Nishioka, R., Ueshige, T., Tobe, Y.: Preparation and evaluation of a chiral stationary phase covalently bound with chiral pseudo-18-crown-6 ether having 1-phenyl-1,2-cyclohexanediol as a chiral unit. J. Chromatogr. A 1078, 35–41 (2005)
Jin, Y.Z., Hirose, K., Nakamura, T., Nishioka, R., Ueshige, T., Tobe, Y.: Preparation and evaluation of a chiral stationary phase covalently bound with a chiral pseudo-18-crown-6 ether having a phenolic hydroxy group for enantiomer separation of amino compounds. J. Chromatogr. A 1129, 201–207 (2006)
Chun, K., Kim, T.H., Lee, O.-S., Hirose, K., Chung, T.D., Chung, D.S., Kim, H.: Structure-selective recognition by voltammetry: enantiomeric determination of amines using azophenolic crowns in aprotic solvent. Anal. Chem. 78, 7597–7600 (2006)
Nakashima, K., Nagaoka, Y., Nakatsuji, S.I., Kaneda, T., Tanigawa, I., Hirose, K., Misumi, S., Akiyama, S.: Fluorescence reactions of “crowned” benzothiazolylphenols with alkali and alkaline earth metal ions and their analytical applications. Bull. Chem. Soc. Jpn. 60, 3219–3223 (1987)
Takagi, M., Nakamura, H.: Analytical application of functionalized crown ether-metal complexes. J. Coord. Chem. 15, 53–82 (1986)
Desilva, A.P., Gunaratne, H.Q.N., Gunnlaugsson, T., Huxley, A.J.M., Mccoy, C.P., Rademacher, J.T., Rice, T.E.: Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97, 1515–1566 (1997)
Hisamoto, H.: Ion-selective optodes: Current developments and future prospects. TrAC Trends Anal. Chem. 18, 513–524 (1999)
Yamauchi, A., Hayashita, T., Nishizawa, S., Watanabe, M., Teramae, N.: Benzo-15-crown-5 fluoroionophore/gamma-cyclodextrin complex with remarkably high potassium ion sensitivity and selectivity in water. J. Am. Chem. Soc. 121, 2319–2320 (1999)
Stradiotto, N.R., Yamanaka, H., Zanoni, M.V.B.: Electrochemical sensors: a powerful tool in analytical chemistry. J. Braz. Chem. Soc. 14, 159–173 (2003)
Hayashita, T., Yamauchi, A., Tong, A.J., Lee, J.C., Smith, B.D., Teramae, N.: Design of supramolecular cyclodextrin complex sensors for ion and molecule recognition in water. J. Incl. Phenom. Macrocycl. Chem. 50, 87–94 (2004)
Uchida, S., Komatsu, Y., Satoh, H., Yajima, S., Kimura, K., Tobe, Y., Sasaki, S., Mizuno, M., Watanabe, Y., Hirose, K.: Properties of dendritic and cyclic thiourea derivatives as neutral carriers for anion sensor. Bunseki Kagaku 53, 943–952 (2004)
Hisaki, I., Sasaki, S.I., Hirose, K., Tobe, Y.: Synthesis and anion-selective complexation of homobenzylic, tripodal thiourea derivatives. Eur. J. Org. Chem., 607–615 (2007)
Faridbod, F., Ganjali, M.R., Dinarvand, R., Norouzi, P., Riahi, S.: Schiff’s bases and crown ethers as supramolecular sensing materials in the construction of potentiometric membrane sensors. Sensors 8, 1645–1703 (2008)
Tsubaki, K.: Colorimetric recognition using functional phenolphthalein derivatives. J. Incl. Phenom. Macrocycl. Chem. 61, 217–225 (2008)
Ozawa, R., Hayashita, T., Matsui, T., Nakayama, C., Yamauchi, A., Suzuki, I.: Effects of cyclodextrins and saccharides on dual fluorescence of N,N-dimethyl-4-aminophenylboronic acid in water. J. Incl. Phenom. Macrocycl. Chem. 60, 253–261 (2008)
Sogah, G.D.Y., Cram, D.J.: Total chromatographic optical resolutions of alpha-amino acid and ester salts through chiral recognition by a host covalently bound to polystyrene resin. J. Am. Chem. Soc. 98, 3038–3041 (1976)
Sogah, G.D.Y., Cram, D.J.: Host–guest complexation.14. Host covalently bound to polystyrene resin for chromatographic resolution of enantiomers of amino acid and ester salts. J. Am. Chem. Soc. 101, 3035–3042 (1979)
Sousa, L.R., Sogah, G.D.Y., Hoffman, D.H., Cram, D.J.: Host–guest complexation. 12. Total optical resolution of amine and amino ester salts by chromatography. J. Am. Chem. Soc. 100, 4569–4576 (1978)
Machida, Y., Nishi, H., Nakamura, K., Nakai, H., Sato, T.: Enantiomeric separation of diols and beta-amino alcohols by chiral stationary phase derived from (R,R)-tartramide. J. Chromatogr. A 757, 73–79 (1997)
Machida, Y., Nishi, H., Nakamura, K., Nakai, H., Sato, T.: Enantiomer separation of amino compounds by a novel chiral stationary phase derived from crown ether. J. Chromatogr. A 805, 85–92 (1998)
Machida, Y., Nishi, H., Nakamura, K.: Separation of the enantiomers of amino and amide compounds on novel chiral stationary phases derived from a crown ether. Chromatographia 49, 621–627 (1999)
Hyun, M.H., Jin, J.S., Lee, W.J.: Liquid chromatographic resolution of racemic amino acids and their derivatives on a new chiral stationary phase based on crown ether. J. Chromatogr. A 822, 155–161 (1998)
Hyun, M.H., Jin, J.S., Koo, H.J., Lee, W.J.: Liquid chromatographic resolution of racemic amines and amino alcohols on a chiral stationary phase derived from crown ether. J. Chromatogr. A 837, 75–82 (1999)
Maier, N.M., Franco, P., Lindner, W.: Separation of enantiomers: needs, challenges, perspectives. J. Chromatogr. A 906, 3–33 (2001)
Hyun, M.H.: Characterization of liquid chromatographic chiral separation on chiral crown ether stationary phases. J. Sep. Sci. 26, 242–250 (2003)
Cram, D.J., Cram, J.M.: Design of complexes between synthetic hosts and organic guests. Acc. Chem. Res. 11, 8–14 (1978)
Cram, D.J., Trueblood, K.N.: Concept, structure, and binding in complexation. Top. Curr. Chem. 98, 43–106 (1981)
Izake, E.L.: Chiral discrimination and enantioselective analysis of drugs: an overview. J. Pharm. Sci. 96, 1659–1676 (2007)
Kubo, Y., Maeda, S., Tokita, S., Kubo, M.: Colorimetric chiral recognition by a molecular sensor. Nature 382, 522–524 (1996)
Van Delden, R.A., Feringa, B.L.: Color indicators of molecular chirality based on doped liquid crystals. Angew. Chem. Int. Ed. 40, 3198–3200 (2001)
Van Delden, R.A., Feringa, B.L.: Colour indicator for enantiomeric excess and assignment of the configuration of the major enantiomer of an amino acid ester. Chem. Commun., 174–175 (2002)
Dietrichbuchecker, C., Sauvage, J.P.: Templated synthesis of interlocked macrocyclic ligands, the catenands. Preparation and characterization of the prototypical bis-30 membered ring system. Tetrahedron 46, 503–512 (1990)
Sauvage, J.P.: Interlacing molecular threads on transition-metals—catenands, catenates, and knots. Acc. Chem. Res. 23, 319–327 (1990)
Sauvage, J.P.: Transition metal-containing rotaxanes and catenanes in motion: toward molecular machines and motors. Acc. Chem. Res. 31, 611–619 (1998)
Dietrichbuchecker, C.O., Sauvage, J.P.: A synthetic molecular trefoil knot. Angew. Chem. Int. Ed. Engl. 28, 189–192 (1989)
Ogino, H.: Relatively high-yield syntheses of rotaxanes—syntheses and properties of compounds consisting of cyclodextrins threaded by alpha, omega-diaminoalkanes coordinated to cobalt(iii) complexes. J. Am. Chem. Soc. 103, 1303–1304 (1981)
Kolchinski, A.G., Busch, D.H., Alcock, N.W.: Gaining control over molecular threading: benefits of second coordination sites and aqueous-organic interfaces in rotaxane synthesis. J. Chem. Soc. Chem. Commun., 1289–1291 (1995)
Simonsen, K.B., Becher, J.: Tetrathiafulvalene thiolates: important synthetic building blocks for macrocyclic and supramolecular chemistry. Synlett 11, 1211–1220 (1997)
Kawasaki, H., Kihara, N., Takata, T.: High yielding and practical synthesis of rotaxanes by acylative end-capping catalyzed by tributylphosphine. Chem. Lett., 1015–1016 (1999)
Zehnder, D.W., Smithrud, D.B.: Facile synthesis of rotaxanes through condensation reactions of dcc-2 rotaxanes. Org. Lett. 3, 2485–2487 (2001)
Furusho, Y., Rajkumar, G.A., Oku, T., Takata, T.: Synthesis of 2 rotaxanes by tritylative endcapping of in situ formed pseudorotaxanes having thiol or hydroxyl functionality on the axle termini. Tetrahedron 58, 6609–6613 (2002)
Schalley, C.A., Weilandt, T., Bruggemann, J., Vogtle, F.: Hydrogen-bond-mediated template synthesis of rotaxanes, catenanes, and knotanes. Templates Chem I 248, 141–200 (2004)
Yoon, I., Narita, M., Shimizu, T., Asakawa, M.: Threading-followed-by-shrinking protocol for the synthesis of a [2] rotaxane incorporating a pd(ii)-salophen moiety. J. Am. Chem. Soc. 126, 16740–16741 (2004)
Arico, F., Badjic, J.D., Cantrill, S.J., Flood, A.H., Leung, K.C.F., Liu, Y., Stoddart, J.F.: Templated synthesis of interlocked molecules. Templates Chem II. 203–259 (2005)
Miljanic, O.S., Dichtel, W.R., Aprahamian, I., Rohde, R.D., Agnew, H.D., Heath, J.R., Stoddart, J.F.: Rotaxanes and catenanes by click chemistry. QSAR Comb. Sci. 26, 1165–1174 (2007)
Narita, M., Yoon, I., Aoyagi, M., Goto, M., Shimizu, T., Asakawa, M.: Transition metal(ii)-salen and -salophen macrocyclic complexes for rotaxane formation: syntheses and crystal structures. Eur. J. Inorg. Chem., 4229–4237 (2007)
Nakazono, K., Oku, T., Takata, T.: Synthesis of rotaxanes consisting of crown ether wheel and sec-ammonium axle under basic condition. Tetrahedron Lett. 48, 3409–3411 (2007)
Haussmann, P.C., Stoddart, J.F.: Synthesizing interlocked molecules dynamically. Chem. Rec. 9, 136–154 (2009)
Harrison, I.T., Harrison, S.: Synthesis of a stable complex of a macrocycle and a threaded chain. J. Am. Chem. Soc. 89, 5723–5724 (1967)
Schill, G., Zollenko, H.: Rotaxane compounds. 1. Liebigs Ann. Chem. 721, 53–74 (1969)
Schill, G., Henschel, R.: Rotaxane compounds. 2. A diansa compound of 5-amino-6-methoxy-4.7-dimethylbenzodioxole as a model of catenanes and rotaxanes. Liebigs Ann. Chem. 731, 113–119 (1970)
Hiratani, K., Suga, J., Nagawa, Y., Houjou, H., Tokuhisa, H., Numata, M., Watanabe, K.: A new synthetic method for rotaxanes via tandem claisen rearrangement, diesterification, and aminolysis. Tetrahedron Lett. 43, 5747–5750 (2002)
Hiratani, K., Kaneyama, M., Nagawa, Y., Koyama, E., Kanesato, M.: Synthesis of [1] rotaxane via covalent bond formation and its unique fluorescent response by energy transfer in the presence of lithium ion. J. Am. Chem. Soc. 126, 13568–13569 (2004)
Kameta, N., Hiratani, K., Nagawa, Y.: A novel synthesis of chiral rotaxanes via covalent bond formation. Chem. Commun., 466–467 (2004)
Nagawa, Y., Suga, J., Hiratani, K., Koyama, E., Kanesato, M.: 3 rotaxane synthesized via covalent bond formation can recognize cations forming a sandwich structure. Chem. Commun., 749–751 (2005)
Hirose, K., Nishihara, K., Harada, N., Nakamura, Y., Masuda, D., Araki, M., Tobe, Y.: Highly selective and high-yielding rotaxane synthesis via aminolysis of prerotaxanes consisting of a ring component and a stopper unit. Org. Lett. 9, 2969–2972 (2007)
Kay, E.R., Leigh, D.A., Zerbetto, F.: Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 46, 72–191 (2007)
Anelli, P.L., Spencer, N., Stoddart, J.F.: A molecular shuttle. J. Am. Chem. Soc. 113, 5131–5133 (1991)
Collier, C.P., Wong, E.W., Belohradsky, M., Raymo, F.M., Stoddart, J.F., Kuekes, P.J., Williams, R.S., Heath, J.R.: Electronically configurable molecular-based logic gates. Science 285, 391–394 (1999)
Wong, E.W., Collier, C.P., Belohradsky, M., Raymo, F.M., Stoddart, J.F., Heath, J.R.: Fabrication and transport properties of single-molecule-thick electrochemical junctions. J. Am. Chem. Soc. 122, 5831–5840 (2000)
Pease, A.R., Jeppesen, J.O., Stoddart, J.F., Luo, Y., Collier, C.P., Heath, J.R.: Switching devices based on interlocked molecules. Acc. Chem. Res. 34, 433–444 (2001)
Collier, C.P., Mattersteig, G., Wong, E.W., Luo, Y., Beverly, K., Sampaio, J., Raymo, F.M., Stoddart, J.F., Heath, J.R.: A [2] catenane-based solid state electronically reconfigurable switch. Science 289, 1172–1175 (2000)
Green, J.E., Wook Choi, J., Boukai, A., Bunimovich, Y., Johnston-Halperin, E., Deionno, E., Luo, Y., Sheriff, B.A., Xu, K., Shik Shin, Y., Tseng, H.R., Stoddart, J.F., Heath, J.R.: A 160-kilobit molecular electronic memory patterned at 1011 bits per square centimetre. Nature 445, 414–417 (2007)
Balzani, V., Venturi, M., Credi, A.: Molecular Devices and Machines—A Journey into the Nanoworld. Wiley-VCH, Weinheim (2003)
Balzani, V., Credi, A., Venturi, M.: Molecular Devices and Machines-Concepts and Perspectives for the Nanoworld. Wiley-VCH, Weinheim (2008)
Kelly, T.R., Bowyer, M.C., Bhaskar, K.V., Bebbington, D., Garcia, A., Lang, F.R., Kim, M.H., Jette, M.P.: A molecular brake. J. Am. Chem. Soc. 116, 3657–3658 (1994)
Lane, A.S., Leigh, D.A., Murphy, A.: Peptide-based molecular shuttles. J. Am. Chem. Soc. 119, 11092–11093 (1997)
Bermudez, V., Capron, N., Gase, T., Gatti, F.G., Kajzar, F., Leigh, D.A., Zerbetto, F., Zhang, S.: Influencing intramolecular motion with an alternating electric field. Nature 406, 608–611 (2000)
Ghosh, P., Federwisch, G., Kogej, M., Schalley, C.A., Haase, D., Saak, W., Lützen, A., Gschwind, R.M.: Controlling the rate of shuttling motions in [2] rotaxanes by electrostatic interactions: a cation as solvent-tunable brake. Org. Biomol. Chem. 3, 2691–2700 (2005)
Coskun, A., Friedman, D.C., Li, H., Patel, K., Khatib, H.A., Stoddart, J.F.: A light-gated STOP-GO molecular shuttle. J. Am. Chem. Soc. 131, 2493–2495 (2009)
Chatterjee, M.N., Kay, E.R., Leigh, D.A.: Beyond switches: ratcheting a particle energetically uphill with a compartmentalized molecular machine. J. Am. Chem. Soc. 128, 4058–4073 (2006)
Jiang, L., Okano, J., Orita, A., Otera, J.: Intermittent molecular shuttle as a binary switch. Angew. Chem. Int. Ed. 43, 2121–2124 (2004)
Chen, N.C., Lai, C.C., Liu, Y.H., Peng, S.M., Chiu, S.H.: Parking and restarting a molecular shuttle in situ. Chem. Eur. J. 14, 2904–2908 (2008)
Yang, J.S., Huang, Y.T., Ho, J.H., Sun, W.T., Huang, H.H., Lin, Y.C., Huang, S.J., Huang, S.L., Lu, H.F., Chao, I.: A pentiptycene-derived light-driven molecular brake. Org. Lett. 10, 2279–2282 (2008)
Hirose, K., Shiba, Y., Ishibashi, K., Doi, Y., Tobe, Y.: An anthracene-based photochromic macrocycle as a key ring component to switch a frequency of threading motion. Chem. Eur. J. 14, 981–986 (2008)
Hirose, K., Shiba, Y., Ishibashi, K., Doi, Y., Tobe, Y.: A shuttling molecular machine with reversible brake function. Chem. Eur. J. 14, 3427–3433 (2008)
Hirose, K., Ishibashi, K., Shiba, Y., Doi, Y., Tobe, Y.: Control of rocking mobility of rotaxanes by size change of stimulus-responsive ring components. Chem. Lett. 36, 810–811 (2007)
Hirose, K., Ishibashi, K., Shiba, Y., Doi, Y., Tobe, Y.: Highly effective and reversible control of rocking rates of rotaxanes by changing size of stimulus-responsive ring components. Chem. Eur. J. 14, 5803–5811 (2008)
Definition of pseudorotaxanes is inclusion complexes in which a thread-like molecule is encircled by one or more ring-like molecules in such a way that the two ends of the thread are projected away from the center of the ring. In a rotaxane, the two ends of the thread are terminated by bulky groups which do not allow the passage of the ring, thus, the two (or more) components are mutually interlocked. The prefix pseudo denotes that in a pseudorotaxane the two components are not interlocked, but instead are free to dissociate because the end groups are small enough to allow passage of the ring
Becker, H.D.: Unimolecular photochemistry of anthracenes. Chem. Rev. 93, 145–172 (1993)
Ashton, P.R., Campbell, P.J., Chrystal, E.J.T., Glink, P.T., Menzer, S., Philp, D., Spencer, N., Stoddart, J.F., Tasker, P.A., Williams, D.J.: Dialkylammonium ion/crown ether complexes: the forerunners of a new family of interlocked molecules. Angew. Chem. Int. Ed. Engl. 34, 1865–1869 (1995)
Glink, P.T., Schiavo, C., Stoddart, J.F., Williams, D.J.: The genesis of a new range of interlocked molecules. Chem. Commun., 1483–1490 (1996)
Ashton, P.R., Chrystal, E.J.T., Glink, P.T., Menzer, S., Schiavo, C., Spencer, N., Stoddart, J.F., Tasker, P.A., White, A.J.P., Williams, D.J.: Pseudorotaxanes formed between secondary dialkylammonium salts and crown ethers. Chem. Eur. J. 2, 709–728 (1996)
Bouas-Laurent, H., Castellan, A., Desvergne, J.P.: From anthracene photodimerization to jaw photochromic materials and photocrowns. Pure Appl. Chem. 52, 2633–2648 (1980)
Moriwaki, F., Ueno, A., Osa, T., Hamada, F., Murai, K.: Photochemical conversion from flexible host to rigid host of a doubly capped γ-cyclodextrin. Chem. Lett. 15, 1865–1868 (1986)
Deng, G., Sakaki, T., Kawahara, Y., Shinkai, S.: Tunable chemical sensors: light-switched ion selective electrodes on the basis of a photoresponsive calix [4] arene. Supramol. Chem. 2, 71–76 (1993)
Tucker, J.H.R., Bouas-Laurent, H., Marsau, P., Riley, S.W., Desvergne, J.P.: A novel crown ether-cryptand photoswitch. Chem. Commun., 1165–1166 (1997)
Yamashita, I., Fujii, M., Kaneda, T., Misumi, S.: Synthetic macrocyclic ligands. 2. Synthesis of a photochromic crown ether. Tetrahedron Lett. 21, 541–544 (1980)
Desvergne, J.P., Lauret, J., Bouas-Laurent, H., Marsau, P., Lahrahar, N., Andrianatoandro, H., Cotrait, M.: Synthesis, X-ray structure, spectroscopic and cation complexation studies of macrocyclic ligands incorporating the 9, 9′-(ethane-1,2-diyl)bis(anthracene) photoactive subunit. Recl. Trav. Chim. Pays-Bas 114, 504–513 (1995)
Becker, H.D., Sanchez, D., Arvidsson, A.: Reductions with diphenylhydroxymethyl radicals. Synthesis of dianthrylethanes and dianthrylethylenes. J. Org. Chem. 44, 4247–4251 (1979)
Matthew, J.C., Fyfe, M.C.T., Stoddart, J.F.: Molecular shuttles by the protecting group approach. J. Org. Chem. 65, 1937–1946 (2000)
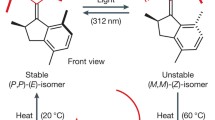
To avoid the decomposition of 1, the solvent for the irradiation with high pressure mercury lamp was limited. For this reaction THF-d 8 and toluene-d 8 were chosen as suitable polar and less polar solvents
The temperature for the VT-NMR measurement was limited because the cycloreversion of the closed-form rotaxane 7c started to occur at higher temperatures during the measurement
Bender, H.L., Farnham, A.G., Guyer, J.W., Apal, P.N., Gibb Jr, T.B.: Ind. Eng. Chem. 44, 1619–1623 (1952)
Arnett, E.M., Wu, C.Y.: Stereoelectronic effects on organic bases. 2. Base strengths of the phenolic ethers. J. Am. Chem. Soc. 82, 5660–5665 (1960)
Hirose, K.: A practical guide for the determination of binding constants. J. Incl. Phenom. Macrocycl. Chem. 39, 193–209 (2001)
The basicities of the phenyl alkyl ethers are affected strongly by the alkyl group due to both inductive and stereoelectronic effects
Line-shape analyses were carried out using (a) P. H. M. Budzelaar, gNMR, Program for simulation of one-dimensional NMR spectra, Adept Scientific plc, Letchworth (United Kingdom), 1999; and a spreadsheet program that we made for determination of the rocking rates on (b) Microsoft Excel 2002, Microsoft Corporation, Tokyo (Japan) (2001)
One of the signals of Hk which appeared at higher field was irradiated at 303 K. However, no change in the signal intensity of the other Hk signal was observed, suggesting that the relaxation time T1 of Hk is longer than the life time of the conformation. T1 was determined by the null point method
Aviram, A., Ratner, M.A.: Molecular rectifiers. Chem. Phys. Lett. 29, 277–283 (1974)
De Silva, A.P., Gunaratne, H.Q.N., Mccoy, C.P.: A molecular photoionic and gate based on fluorescent signalling. Nature 364, 42–44 (1993)
Zheng, X., Mulcahy, M.E., Horinek, D., Galeotti, F., Magnera, T.F., Michl, J.: Dipolar and nonpolar altitudinal molecular rotors mounted on an Au(111) surface. J. Am. Chem. Soc. 126, 4540–4542 (2004)
Acknowledgements
The author thanks the Organizing Committee of Host–Guest and Supramolecular Chemistry Society, Japan for giving him the HGCS Japan Award of Excellence 2009 and the opportunity to write this review article. He acknowledges all collaborators for their efforts. He especially thanks Prof. Yoshito Tobe for his suggestions, discussions, and encouragements. This work was partly supported by the Izumi Science and Technology Foundation and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
This is a paper selected for ‘‘HGCS Japan Award of Excellence 2009’’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirose, K. Molecular brake systems controlled by light and heat. J Incl Phenom Macrocycl Chem 68, 1–24 (2010). https://doi.org/10.1007/s10847-010-9748-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9748-x