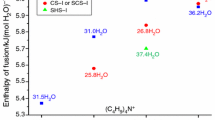
Abstract
Temperatures of hydrate decomposition were measured by means of the differential thermal analysis at a pressures up to 800–900 Mpa in the systems: cross-linked tetrabutylammonium polyacrylate–water and cross-linked tetrabutylammonium polyacrylate–water–noble gas (He, Ne, Ar, Kr, Xe). The effect of the deformation of D- cavities of the hydrates on the temperature of their decomposition is discussed on the basis of the experimental data.



Similar content being viewed by others

References
Jeffrey, G.A.: Hydrate inclusion compounds. In: Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Vogtle, F. (eds.) Comprehensive Supramolecular Chemistry, vol. 6, pp. 757–788. Pergamon Press, Oxford (1996)
Dyadin, Y.A., Udachin, K.A.: Clathrate polyhydrates of peralkylonium salts and their analogs. J. Struct. Chem. 28(3), 394–432 (1987)
Bogatyryov, V.L.: Clathrate-forming ion exchangers. In: Muraviev, D., Gorshkov, V., Warshawsky, W. (eds.) Ion Exchange, vol. 1, p. 223. Marcel Dekker, New York (1998)
Terekhova, I.S., Bogatyryov, V.L., Dyadin, Yu.A.: Clathrate hydrates of cross-linked tetraisoamylammonium polyacrylates. J. Supramol. Chem. 2, 393–399 (2002)
Nakayama, H.: Hydrates of organic compounds. XIII. The confirmation of the formation of clathrate-like hydrates of tetrabutylammonium and of tetraisopentylammonium polyacrylates. Bull. Chem. Soc. Jpn. 60, 2319–2326 (1987)
Bella, J., Brodsky, B., Berman, H.: Hydration structure of a collagen peptide. Structure 3(9), 893–906 (1995)
Nakasako, M.: Structural characteristics in protein hydration investigated by cryogenic X-ray crystal structure analyses. J. Biolog. Phys. 28, 129–137 (2002)
Shataeva, L.K., Kuznetsova, N.N., El’kin, G.E.: Karboxyl’nye kationity v biologii (The use of Carboxylic Cation-Exchange Resins in Biology). Nauka, Leningrad (1979). (in Russian)
Chapoy, A., Anderson, R., Tohidi, B.: Low pressure molecular hydrogen storage in semi-clathrate hydrates of quaternary ammonium compounds. J. Am. Chem. Soc. 129, 746–747 (2007)
Skiba, S.S., Terekhova, I.S., Larionov, E.G., Manakov, A.Y.: Incorparation of gas molecules into the frameworks of clathrate hydrates of ion-exhange resins in tetraalkylammonium form. Mendeleev Commun. 18, 126–127 (2008)
Terekhova, I.S., Manakov, A.Yu., Feklistov, V.V., Dyadin, Yu.A., Komarov, V.Yu., Naumov, D.Yu.: X-ray powder diffraction studies of polyhydrates of cross-linked tetraisoamylammonium polyacrylates. J. Incl. Phenom. Macro. Chem. 52, 207–211 (2005)
Udachin, K.A., Ripmeester, J.A.: A polymer guest transforms clathrate cages into channels: the single-crystal X-ray structure of tetra-n-butylammonium polyacrylate hydrate, nBu4NPA·40 H2O. Angew. Chem. Int. Ed. 38(13/14), 1983–1984 (1999)
Soldatov, D.V., Suwinska, K., Terekhova, I.S., Manakov, A.Y.: Structural study of hydrate compounds of polyacrylate resins in tetraisoamylammonium form. crystal structure of a clathrate hydrate of linear polyacrylate in tetraisoamylammonium form. J. Struct. Chem. 49(4), 712–718 (2008)
Glew, D.N., Mak, H.D., Rath, N.S.: Aqueous non-electrolyte solutions: Part VII. Water shell stabilization by interstitial nonelectrolytes. In: Covington, A.K., Jones, P. (eds.) Hydrogen-Bonded Solvent Systems, pp. 185–193. Taylor & Francis Ltd, London (1968)
Dyadin, Y.A., Larionov, E.G., Manakov, A.Y., Zhurko, F.V.: Double clathrate hydrate of tetrahydrofuran and xenon at pressures up to 15 kbar. Mendeleev Commun. 2, 80–81 (1999)
Dyadin, A.Yu., Larionov, E.G., Mirinskij, D.S., Mikina, T.V., Aladko, E.Ya., Starostina, L.I.: Phase diagram of the Xe-H2O system up to 15 kbar. J. Incl. Phenom. 28, 271–285 (1997)
Dyadin, Yu.A., Larionov, E.G., Mikina, T.V., Starostina, L.I.: Clathrate hydrate of xenon at high pressure. Mendeleev Commun. 6, 44–45 (1996)
Dyadin, A.Yu., Zelenin, Yu.M., Bezuglov, S.G., Bondaryuk, I.V.: Clathrate hydrates and phase diagram of the system water-acetone under pressure to 10 kbar. Izv Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk 7, 70 (1978)
Larionov, E.G., Manakov, A.Y., Zhurko, F.V., Dyadin, Y.A.: Double clathrate hydrate CS-II at pressures up to 15 kbar. J. Struct. Chem. 41(3), 476–482 (2000)
Larionov, E.G., Zhurko, F.V., Dyadin, Y.A.: Gas-hydrate packing and stability at high pressures. J. Struct. Chem. 43(6), 985–989 (2002)
Batsanov, S.S.: Atomic radii of elements. Zhurnal Neorganicheskoi Khimii 36(12), 3015–3047 (1991)
Acknowledgements
This work was financially supported by the Integration project of the Presidium of SB RAS No 62.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aladko, E.Y., Larionov, E.G., Manakov, A.Y. et al. Double clathrate hydrates of cross-linked tetrabutylammonium polyacrylate and noble gases at high pressures. J Incl Phenom Macrocycl Chem 67, 13–18 (2010). https://doi.org/10.1007/s10847-009-9662-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9662-2