Abstract
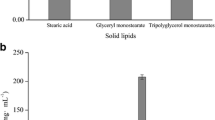
Solid lipid nanoparticles (SLN) made of different triglycerides (TG) in the presence and in the absence of various modified α- and γ-cyclodextrins (CD) were prepared by the solvent injection technique. A new synthesis of lipophilic derivatives of γ- CD was developed in this work. Curcumin (CU), a natural polyphenol with antitumor, antioxidant and anti-inflammatory properties, was used as model drug. SLNs mean sizes were in the 250–800 nm range and afforded CU entrapment efficiency in the 12–85% range. The presence of CD derivatives with almost the same chain length of TG induced an improvement of nanoparticle characteristics decreasing mean size values and increasing CU entrapment efficiency. A significant reduction in CU photodegradation was noted only when the drug was vehicled in tristearin-SLN, which became less pronounced in the presence of CD-derivatives, determining a loss in photoprotection. The hydrolytic stability of curcumin was highly improved by drug loading in tristearin-SLN, and only slightly by loading it in tricaprin-SLN, and this seemed not to be influenced by the presence of CD derivatives. Skin uptake studies revealed an increase in CU skin accumulation when CU was loaded in SLN obtained with all CD derivatives, particularly with most lipophilic one.









Similar content being viewed by others
References
Ammon, H.P., Wahl, M.A.: Pharmacology of Curcuma Longa. Planta Med. 57, 1–7 (1991)
Maheshwari, R.K., Singh, A.K., Gaddipati, J., Srimal, R.C.: Multiple biological activities of curcumin: a short review. Life Sci. 78, 2081–2087 (2006)
Aggarwal, B.B., Kumar, A., Bharti, A.C.: Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 23, 363–398 (2003)
Bush, J.A., Cheung, K.-J.J., Li, G.: Curcumin induces apoptosis in human melanoma cells through a fas receptor/caspase-8 pathway independent of p53. Exp. Cell Res. 271, 305–314 (2001)
Lin, J.K., Pan, M.H., Shiau, S.Y.L.: Recent studies on the biofunctions and biotransformations of curcumin. Biofactors 13, 153–158 (2000)
Pfeiffer, E., Hohle, S., Solyom, A., Metzler, M.: Studies on the stability of turmeric constituents. J. Food Eng. 56, 257–259 (2003)
Anand, P., Kunnumakkara, A.B., Newman, R.A., Aggarwal, B.B.: Bioavailability of curcumin: problems and promises. Mol. Pharm. 4, 807–818 (2007)
Tiyaboonchai, W., Tungpradit, W., Plianbangchang, P.: Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int. J. Pharm. 337, 299–306 (2007)
Li, L., Braiteh, F.S., Kurzrock, R.: Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling and angiogenesis. Cancer 104(6), 1322–1331 (2005)
Suresh, D., Srinivasan, K.: Studies on the in vitro absorption of spice principles-curcumin, capsaicin, and piperine in rat intestines. Food Chem. Toxicol. 45, 1437–1442 (2007)
Ma, Z., Haddadi, A., Molavi, O., Lavasanifar, A., Lai, R., Samuel, J.: Micelles of poly(ethylene oxide)-β-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J. Biomed. Mater. Res. A 86, 300–310 (2008)
Tønnesen, H., Masson, M., Loftsson, T.: Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int. J. Pharm. 244, 127–135 (2002)
Roux, M., Perly, B., Djedaïni-Pilard, F.: Self assemblies of amphiphilic cyclodextrins. Eur. Biophys. J. 36, 861–867 (2007)
Vico, R.V., Silva, O.F., De Rossi, R.H., Maggio, B.: Molecular organization, structural orientation, and surface topography of monoacylated β-cyclodextrins in monolayers at the air-aqueous interface. Langmuir 24, 7867–7874 (2008)
Trotta, F., Moraglio, G., Marzona, M., Maritano, S.: Acyclic carbonates of β-cyclodextrin. Gazzetta Chimica Italiana 123, 559–562 (1993)
Franz, T.J.: The percutaneous absorption on the relevance of in vitro data. J. Invest. Dermatol. 190–195 (1975)
Beck, H., Bracher, M.: Protocollo standard: assorbimento/penetrazione percutaneo/a “in vitro” con pelle suina. Acta Technologiae et Legis Medicamenti 2, 123–134 (1991)
Tong, L.H., Hou, Z.J., Inoue, Y., Tai, A.: Molecular recognition by modified cyclodextrins. Inclusion complexation of β-cyclodextrin 6-O-monobenzoate with acyclic and cyclic hydrocarbons. J. Chem. Soc. Perkin Trans. 2, 1253–1257 (1992)
Rao, C.T., Lindberg, B., Lindberg, J., Pitha, J.: Substitution in beta-cyclodextrin directed by basicity: preparation of 2-O-and 6-O-[(R)-and (S)-2-hydroxypropyl] derivatives. J. Org. Chem. 56, 1327–1329 (1991)
Xiao, Y., Wu, Q., Wanga, N., Lin, X.: Regioselective monoacylation of cyclomaltoheptaose at the C-2 secondary hydroxyl groups by the alkaline protease from Bacillus subtilis in nonaqueous media. Carbohydr. Res. 339, 1279–1283 (2004)
Cavalli, R., Trotta, F., Carlotti, M.E., Possetti, B., Trotta, M.: Nanoparticles derived from amphiphilic γ-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 57, 657–661 (2007)
Siekmann, B., Westesen, K.: Thermoanalysis of the recrystallization process of melt-homogenized glyceride nanoparticles. Colloids Surf. B 3, 159–175 (1994)
Weber, W.M., Hunsaker, L.A., Abcouwer, S.F., Deck, L.M., Vander Jagt, D.L.: Auto-oxidant activities of curcumin and related enones. Bioorg. Med. Chem. 13, 3811–3820 (2005)
Srinivasan, M., Rajendra Prasad, N., Menon, V.P.: Protective effect of curcumin on γ-radiation induced DNA damage and lipid peroxidation in cultured human lymphocytes. Mutat. Res. 611, 96–103 (2006)
Müller, R.H., Radtke, M., Wissing, S.A.: Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 54, 131–155 (2002)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 85, 1017–1024 (1996)
Tønnesen, H.H., Karlsen, J.: Studies on curcumin and curcuminoids. VI. Kinetics of curcumin degradation in aqueous solution. Z. Lebensm.-Unters.-Forsch. 180, 402–404 (1985)
Tønnesen, H.H., Karlsen, J.: Studies on curcumin and curcuminoids. V. Alkaline degradation of curcumin. Z. Lebensm.-Unters.-Forsch. 180, 132–134 (1985)
Shishodia, S., Chaturvedi, M.M., Aggarwal, B.B.: Role of Curcumin in Cancer Therapy. Curr. Probl. Cancer 31, 243–305 (2007)
Huang, M.T., Ma, W., Yen, P., Xie, J.G., Han, J., Frenkel, K.: Inhibitory effects of topical application of low doses of curcumin on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion and oxidized DNA bases in mouse epidermis. Carcinogenesis 18, 83–88 (1997)
Azuine, M.A., Kayal, J.J., Bhide, S.V.: Protective role of aqueous turmeric extract against mutagenicity of direct-acting carcinogens as well as benzo-pyrene induced genotoxicity and carcinogenicity. J. Cancer Res. Clin. Oncol. 118, 447–452 (1992)
Acknowledgments
This work was supported by a grant from the Italian Government (MIUR, Cofin 2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chirio, D., Gallarate, M., Trotta, M. et al. Influence of α- and γ- cyclodextrin lipophilic derivatives on curcumin-loaded SLN. J Incl Phenom Macrocycl Chem 65, 391–402 (2009). https://doi.org/10.1007/s10847-009-9597-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9597-7