Abstract
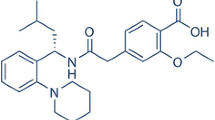
A tricyclic, piperidine derivative of antihistamines, loratadine, which belongs in class II of the Biopharmaceutical Classification System, was investigated. It is an ionizable drug, whose solubility depends on the gastrointestinal pH, and the bioavailability is therefore very variable. The aim of this work was to enhance the dissolution and make the solubility of loratadine independent of pH. Inclusion complexes were prepared between loratadine and dimethyl-β-cyclodextrin in two different molar ratios by three techniques (physical mixing, kneading and spray-drying). The formation and physicochemical properties of the inclusion complexes were investigated by means of dissolution tests, thermal analysis and Fourier Transform Infrared spectroscopy. The instrumental examinations proved the presence of partial or total complexes depending on the preparation method and molar ratio, which resulted in better dissolution. For some compositions and preparation methods, the application of this cyclodextrin made the solubility of loratadine independent of pH.






Similar content being viewed by others
References
Merisko-Liversidge, E., Liversidge, G.G., Cooper, E.R.: Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 18, 113–120 (2003). doi:10.1016/S0928-0987(02)00251-8
Loftsson, T., Jarho, P., Másson, M., Järvinen, T.: Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2, 335–351 (2005). doi:10.1517/17425247.2.1.335
Higuchi, T., Connors, K.A.: Phase-solubility techniques. Adv. Anal. Chem. Instrum. 4, 117–212 (1965)
Pouton, C.W.: Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 29, 278–287 (2006). doi:10.1016/j.ejps.2006.04.016
Bhattachar, S.N., Deschenes, L.A., Wesley, J.A.: Solubility: it’s not just for physical chemists. Drug Discov. Today 11, 1012–1018 (2006). doi:10.1016/j.drudis.2006.09.002
Hörter, D., Dressman, J.B.: Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv. Drug Deliv. Rev. 46, 75–87 (2001). doi:10.1016/S0169-409X(00)00130-7
Dressman, J.B., Amidon, G.L., Reppas, C., Shah, V.P.: Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm. Res. 15, 11–22 (1998). doi:10.1023/A:1011984216775
Zimmermann, T., Yeates, R.A., Laufen, H., Pfaff, G., Wildfeuer, A.: Influence of concomitant food intake on the oral absorption of two triazole antifungal agents, itraconazole and fluconazole. Eur. J. Clin. Pharmacol. 46, 147–150 (1994). doi:10.1007/BF00199879
Dashevsky, A., Kolter, K., Bodmeier, R.: pH-independent release of a basic drug from pellets coated with the extended release polymer dispersion Kollicoatw SR 30 D and the enteric polymer dispersion Kollicoatw MAE 30 DP. Eur. J. Pharm. Biopharm. 58, 45–49 (2004). doi:10.1016/j.ejpb.2004.03.013
Estelle, F., Simons, R.: Comparative pharmacology of H1 antihistamines: clinical relevance. Am. J. Med. 113(9), 38–46 (2002)
Khan, M.Z., Raušl, D., Zanoški, R., Zidar, S., Mikulčić, J.H., Krizmanić, L., Eškinja, M., Mildner, B., Knežević, Z.: Classification of loratadine based on the biopharmaceutics drug classification concept and possible in vitro–in vivo correlation. Biol. Pharm. Bull. 27(10), 1630–1635 (2004). doi:10.1248/bpb.27.1630
Omar, L., El-Barghouthi, M.I., Masoud, N.A., Abdoh, A.A., Al Omari, M.M., Zughul, M.B., Badwan, A.A.: Inclusion complexation of loratadine with natural and modified cyclodextrins: phase solubility and thermodynamic studies. J. Solution Chem. 36, 605–616 (2007). doi:10.1007/s10953-007-9136-3
ter Laak, A.M., Tsai, R.S., Donné-Op den Kelder, G.M., Carrupt, P.-A., Testa, B., Timmermann, H.: Lipophilicity and hydrogen-bonding capacity of H1-antihistaminic agents in relation to their central sedative side-effects. Eur. J. Pharm. Sci. 2, 373–384 (1994). doi:10.1016/0928-0987(94)00065-4
Moffat, A.C., Osselton, M.D., Widdop, B.: Clarke’s Analysis of Drugs and Poisons, 3rd ed. edn. Pharmaceutical Press, London (2004)
Caliaro, G.A., Herbots, C.A.: Determination of pKa values of basic new drug substances by CE. J. Pharm. Biomed. Anal. 26, 427–434 (2001). doi:10.1016/S0731-7085(01)00423-X
Peterson, M.L., Hickey, M.B., Zaworotko, M.J., Almarsson, Ö.: Expanding the scope of crystal form evaluation in pharmaceutical science. J. Pharm. Pharm. Sci. 9, 317–326 (2006)
Carrier, R.L., Miller, L.A., Ahmed, I.: The utility of cyclodextrins for enhancing oral bioavailability. J. Control. Release 123, 78–99 (2007). doi:10.1016/j.jconrel.2007.07.018
Badr-Eldin, S.M., Elkheshen, S.A., Ghorab, M.M.: Inclusion complexes of tadalafil with natural and chemically modified β-cyclodextrins. I: preparation and in vitro evaluation. Eur. J. Pharm. Biopharm. 70, 819–827 (2008). doi:10.1016/j.ejpb.2008.06.024
Kata, M., Ambrus, R., Aigner, Z.: Preparation and investigation of inclusion complexes containing nifluminic acid and cyclodextrins. J. Incl. Phenom. 44, 123–126 (2002). doi:10.1023/A:1023074025175
Hassan, H.B., Kata, M., Erős, I., Aigner, Z.: Preparation and investigation of inclusion complexes containing gemfibrozil and DIMEB. J. Incl. Phenom. 50, 219–225 (2004). doi:10.1007/s10847-004-3124-7
Amidon, G.L., Lennernas, H., Shah, V.P., Crison, J.R.: A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12, 413–420 (1995). doi:10.1023/A:1016212804288
Lipinski, C.A., Lombardo, F., Dominy, B.W., Feeney, P.J.: Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001). doi:10.1016/S0169-409X(00)00129-0
Marttin, E., Verhoef, J.C., Merkus, F.W.: Efficacy, safety and mechanism of cyclodextrins as absorption enhancers in nasal delivery of peptide and protein drugs. J. Drug Target. 6, 17–36 (1998)
Loftsson, T., Brewster, M.E., Másson, M.: Role of cyclodextrins in improving oral drug delivery. Am. J. Drug Deliv. 2, 261–275 (2004). doi:10.2165/00137696-200402040-00006
Desiraju, G.R.: Crystal and co-crystal. Cryst. Eng. Commun. 5, 466–467 (2003). doi:10.1039/b313552g
Acknowledgements
This work was performed with the support of a Sanofi-Aventis Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nacsa, Á., Berkesi, O., Szabó-Révész, P. et al. Achievement of pH-independence of poorly-soluble, ionizable loratadine by inclusion complex formation with dimethyl-β-cyclodextrin. J Incl Phenom Macrocycl Chem 64, 249–254 (2009). https://doi.org/10.1007/s10847-009-9558-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9558-1