Abstract
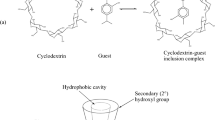
Cyclodextrins (CDs) are cyclic oligosaccharides that form inclusion complexes with lipophilic molecules through their hydrophobic central cavity. In this study, the effect of α-CD, hydroxylpropyl-β-CD (HP-β-CD) and mixtures of these two CDs on the aqueous solubility of cyclosporine A (CyA) was investigated. Infrared spectroscopy and thermal analysis were used to confirm CyA-CD complex formation. CyA aqueous solubility was increased by 10 and 80 fold in the presence of α-CD and HP β-CD, respectively. The phase-solubility profile for HP-β-CD was linear while that for α-CD had positive deviation from linearity. In the presence of constant concentration of α-CD (15% w/v), aqueous solubility of CyA was further increased upon addition of HP-β-CD up to a concentration of 20% w/v. At higher HP-β-CD concentrations, aqueous solubility of CyA was observed to decrease. Addition of sodium acetate (up to 5% w/v) to aqueous solutions containing 20% w/v HP-β-CD and increasing concentrations of α-CD resulted in a significant reduction in CyA solubility. Complex formation between CyA and both α-CD and HP-β-CD was confirmed by differential scanning calorimetry (DSC). No significant changes were observed in the IR spectra of either CyA or CD following complex formation suggesting chemical interaction between CyA and the CD was unlikely. Phase-solubility studies showed that α-CD had a much greater effect on the solubility of CyA than HP-β-CD. Addition of HP-β-CD to aqueous solutions of α-CD affected the solubility of CyA in these systems. A mixture of 15% w/v α-CD and 20% w/v HP-β-CD was optimal for increasing aqueous solubility of CyA.






Similar content being viewed by others
References
Allison, A.C.: Immunosuppressive drugs: The first 50 years and a glance forward. Immunopharmacology 47, 63–83 (2000)
Kawabata, T.T., Munson, A.E.: Immunopharmacology. In: Brody, T.M., Larner, J., Minneman, K.P. (eds.) Human pharmacology, molecular to clinical, 3rd edn. pp. 599–637. Mosby, USA (1998)
Lake, D.F., Akporiaye, E.T., Hersh, E.M.: Immunopharmacology. In: Katzung, B.G. (ed.) Basic & clinical pharmacology, 8th edn. pp. 959–986. The McGraw-Hill Companies, USA (2001)
Ptachcinski, R.J., Venkataramanan, R., Burckart G.J.: Clinical pharmacokinetics of cyclosporine. Clin. Pharmacokinet. 11(2), 107–132 (1986)
Lee, E., Choi, H., Lee, S., Kim, C.: Bioavailability of cyclosporine A dispersed in sodium lauryl sulfate dextrin based Solid microspheresInt. J. Pharm. 218, 125–131 (2001)
Guo, J., Chen, Y., Ping, Q.: 6) Pharmacokinetic behavior of cyclosporine A in rabbits by oral administration of lecithin and Sandimmun Neoral. Int. J. Pharm. 216, 17–21 (2001)
Al-Meshal, M.A., Khadir, S.H., Bayomi, M.A., Al-Angary, A.A.: Oral administration of liposomes containing cyclosporine: A pharmacokinetic studyInt. J. Pharm. 168, 163–168 (1998)
Gelderblom, H., Verweij, J., Nooter, K., Sparreboom, A.: 8) Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 37, 1590–1598 (2001)
Loftsson, T., O’Fee, R.: Cyclodextrins in solid dosage forms. Business Briefing: Pharmatech. 176–180 (2003)
Davis, M.E., Brewster, M.E.:Cyclodextrin-based pharmaceutics: Past, present and future. Nature Rev. 3, 1023–1035 (2004)
Loftsson, T., Masson, M.: Cyclodextrins in topical drug formulations: Theory and practice. Int. J. Pharm. 225, 15–30 (2001)
Martin Del Valle, E.M.: Cyclodextrins and their uses: A review. Process Biochem. 39, 1033–1046 (2004)
Filipovic-Grcic, J., Voinovich, D., Moneghini, M., Becirevic-Lacan, M., Magarotto, L., Jalsenjak, I.: Chitosan microspheres with hydrocortisone and hydrocortisone–hydroxypropyl-β-cyclodextrin inclusion complex. Eur. J. Pharm. Biopharm. 9, 373–379 (2000)
Filipovic-Grcic, J., Becirevic-Lacan, M., Skalko, N., Jalsenjak, I.: Chitosan microspheres of nifedipine and nifedipine-cyclodextrin inclusion complexes. Int. J. Pharm. 135, 183–190 (1996)
Loftsson, T., Matthiasson, K., Masson, M.: The effect of organic salts on cyclodextrin solubilization of drugs. Int. J. Pharm. 262, 101–107 (2003)
Ran, Y., Zhao, L., Xu, Q., Yalkowsky, S.H.: 16) Solubilization of cyclosporine A. AAPS PharmSciTech 20(1), article 2 (2001)
Loftsson, T., Magnudottir, A., Masson, M., Sigurjonsdottir, J.F.: Self-association and cyclodextrin solubilization of drugs. J. Pharm. Sci. 91(11), 2307–2316 (2002)
Fromming, K., Szejtli, J.: CDs in Pharmacy. Kluwer Academic Publishers, Netherlands (1994) pp. 1–32
Suzuki, M., Tasutsui, M., Ohamori, H.: NMR study of the self-assembly of an azo dye-cyclomaltooctanose (γ-cyclodextrin) complex. Carbohyd. Res. 264, 233–230 (1994)
Fukaya, H., Iimura, A., Hoshiko, K., Fuyumuro, T., Noji, S., Nabeshima, T.: A cyclosporin A/maltosyl-α-cyclodextrin complex for inhalation therapy of asthma. Eur. Respir. J. 22(2), 213–219 (2003)
Zhang, A., Liu, W., Wang L., Wen Y.: Characterization of inclusion complexation between fenoxaprop-p-ethyl and cyclodextrin. J. Agr. Food Chem. 53(18), 7193–7197 (2005)
Liu, L., Zhu, S.: 22) Preparation and characterization of inclusion complexes of prazosin hydrochloride with beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin. J. Pharm. Biomed. Anal. 40(1), 122–127 (2006)
Anselmi, C., Centini, M., Ricci, M., Buonocore, A., Granata, P., Tsuno, T., Facino, R.M.: 23) Analytical characterization of a ferulic acid/γ-cyclodextrin inclusion complex. J. Pharm. Biomed. Anal. 40(4), 875–881 (2006)
Zerrouk, N., Gine`s Dorado, J.M., Arnaud, P., Chemtob, C.: 24) Physical characteristics of inclusion compounds of 5-ASA in α and β cyclodextrins. Int. J. Pharm. 171, 19–29 (1998)
Calabrò, M.L., Tommasini, S., Donato, P., Raneri, D., Stancanelli, R., Ficarra, P., Costa, C., Catania, S., Rustichelli, C., Gamberini, G.: 25) Effects of α- and β-cyclodextrin complexation on the physicochemical properties and antioxidant activity of some 3-hydroxyflavones. J. Pharm. Biomed. Anal. 35, 365–377 (2004)
Luciana, M.A, Fraceto, L.F., Santana, M.H.A., Pertinhez, T.A., Junior, S.O., de Paula, E.: 26) Physico-chemical characterization of benzocaine-β-cyclodextrin inclusion complexes. J. Pharm. Biomed. Anal. 39(5), 956–963 (2005)
Li, N., Zhang, Y., Wu, Y., Xiong, X., Zhang, Y.: 27) Inclusion complex of trimethoprim with β-cyclodextrin. J. Pharm. Biomed. Anal. 39(3–4), 824–829 (2005)
Acknowledgements
This work was supported financially by a research grant from the Vice Chancellor for Research of Mashhad University of Medical Sciences, Mashhad, I. R. Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malaekeh-Nikouei, B., Nassirli, H. & Davies, N. Enhancement of cyclosporine aqueous solubility using α- and hydroxypropyl β-cyclodextrin mixtures. J Incl Phenom Macrocycl Chem 59, 245–250 (2007). https://doi.org/10.1007/s10847-007-9321-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-007-9321-4