Abstract
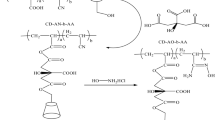
In this study, a new aqueous insoluble ionic β-cyclodextrin polymer (PYR), synthesized by reaction of β-cyclodextrin with pyromellitic anhydride [1], is characterized by IR spectroscopy, showing typical cyclodextrin and carboxylic absorptions. pH-metric titrations of the acidic functions with standard NaOH solutions followed by a refinement of protonation constants, with specific software for equilibrium in solution, have been performed. Through this approach, the pK a values of the functional groups have been calculated. The complexation capabilities of PYR towards metal ions [Al(III), Mn(II), Co(II), Ni(II), Cu(II), Zn(II), Pd(II), Cd(II), Pt(IV), Tl(I), and U(IV)] have been evaluated in aqueous solution (pH 3–5). The retention is mainly pH dependent and higher than 70% for Al(III), Mn(II), Co(II), Ni(II), Cu(II), Zn(II), Pd(II), Cd(II) and U(IV). For Tl(I) and Pt(IV) the retention is about 60% and 40% respectively.


Similar content being viewed by others
References
Trotta, F., Tumiatti, W., Vallero, R.: Italian patent N. MI2004A000614
Larsen, K.L.: Large cyclodextrins. J. Inclusion. Phenom. Mol. Recognit. Chem. 43, 1–13 (2002)
Bender, M.L., Komiyama M.: Cyclodextrin Chemistry, Springer-Verlag, Berlin Heidelberg New York (1978) pp. 2–8
Martin Del Valle, E.M.: Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
Juvancz, Z., Szejtli J.: The role of cyclodextrins in chiral selective chromatography. TrAC, Trends Anal. Chem. 21–5, 379–388 (2002)
Liu, Y., Chang, X., Hu, X., Guo, Y., Meng, S., Wang, F.: Highly selective determination of total mercury(II) sub microgram per liter by β-cyclodextrin polymer solid-phase spectrophotometry using 1,3-di-(4-nitrodiazoamino)-benzene. Anal. Chim. Acta 532, 121–128 (2002)
Bhaskar, M., Aruna, P., Ganesh, J., Rama, J., Radhakrishnan, G.: β-Cyclodextrin-polyurethane polymer as solid phase extraction material for the analysis of carcinogenic aromatic amines. Anal. Chim. Acta 509, 39–45 (2004)
Asanuma, H., Hishiya, T., Komiyama, M.: Efficient separation of hydrophobic molecules by molecularly imprinted cyclodextrin polymers. J. Incl. Phenom. Macrocycl. Chem. 50, 51–55 (2004)
He, H.-B., Zhang, W.-N., Da, S.-L., Feng, Y.-Q.: Preparation and characterization of a magnesia–zirconia stationary phase modified with β-cyclodextrin for reversed-phase high-performance liquid chromatography. Anal. Chim. Acta 513, 481–492 (2004)
Reece, D.A., Ralph, S.F., Wallace, G.G.: Metal transport studies on inherently conducting polymer membranes containing cyclodextrin dopants. J. Membr. Sci. 249, 9–20 (2005)
De Stefano, C., Mineo, P., Rigano, C., Sammartano, S.: Ionic strength dependence of formation constants. XVII. The calculation of equilibrium concentrations and formation constants. Ann. Chim. (Rome) 83, 243–277 (1993)
De Stefano, C., Princi, P., Rigano, C., Sammartano, S.: Computer analysis of equilibrium data in solution. ESAB2M: an improved version of the ESAB program. Ann. Chim. (Rome) 77, 643–675 (1987)
Soldatov, V.S.: A simple method for the determination of the acidity parameters of ion exchangers. Reac. Func. Polymers 46, 55–58 (2000)
Soldatov, V.S., Sosinovich, Z.I., Mironova, T.V.: Acid–base properties of ion exchangers. II. Determination of the acidity parameters of ion exchangers with arbitrary functionality. Reac. Func. Polymers 58, 13–26 (2004)
Zelano, V., Daniele, P.G., Berto, S., Ginepro, M., Laurenti, E., Prenesti, E.: Metals ions distribution between water and river sediment: speciation model and spectroscopic validation. Ann. Chim. (Rome) 96, 1–11 (2006)
Smith, R.M., Martell, A.E., Motekaitis, R.J.: NIST Critical Selected Stability Constant of Metal Complexes Databases, version 6.0 (2002)
Daniele, P.G., Rigano, C., Sammartano, S.: Ionic strength dependence of formation constants. Alkali metal complexes of ethylenediaminetetraacetate nitrilotriacetate, diphosphate, and tripolyphosphate in aqueous solution. Anal. Chem. 57, 2956–2960 (1985)
Acknowledgements
The financial contribution (PRIN 2004) from MIUR (Ministero dell’Istruzione, dell’Università e della Ricerca), Italy is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berto, S., Bruzzoniti, M.C., Cavalli, R. et al. Synthesis of new ionic β-cyclodextrin polymers and characterization of their heavy metals retention. J Incl Phenom Macrocycl Chem 57, 631–636 (2007). https://doi.org/10.1007/s10847-006-9273-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-006-9273-0