Abstract
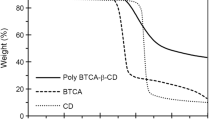
A new aqueous insoluble ionic β-cyclodextrin polymer (PYR) has been synthesized and a potentiometric study of the binary Cu(II)-PYR system is performed to calculate the complexation constants (as logβ in heterogeneous medium). The mathematical processing of the pH-metric data gave the formation constants of Cu(II) complexes and the related species distributions. The model is compatible with the presence of five complex species in the range of pH 2.5–7. Stoichiometry indicates the probable involvement of the alcoholate functionalities of the ligand in the complexation. The capacity of the polymer with respect to metal ions retention is evaluated for both Cu(II) and Cd(II) (chosen as target probes). The possibility to recover the sorbed Cd(II) is also tested by using acidic pH solutions. A complete recovery is obtained and the stability of the polymer is verified over ten steps of retention and desorption. To understand the complexation mechanism involved, two other cyclodextrin-based polymers are synthesized which are characterized by the presence of naphthalic dicarboxylic and carbonate groups as spacers. Their interactions with Cu(II) or Cd(II) are studied. Since the β-cyclodextrin polycarbonate polymer does not have acidic groups on the spacer, it is interesting to compare metal ions retention between this material, which does not present a real cation exchange site, and PYR.






Similar content being viewed by others
References
Berto, S., Bruzzoniti, M.C., Cavalli, R., Perrachon, D., Prenesti, E., Sarzanini, C., Trotta, F., Tumiatti, W.: Synthesis of new ionic β-cyclodextrin polymers and characterization of their heavy metals retention. J. Incl. Phenom. Macrocycl. Chem. (2007). DOI: 10.1007/s10847-006-9273-0
Martin Del Valle, E.M.: Cyclodextrins and their use: a review. Process Biochem. 39, 1033–1046 (2004)
Duchêne, D., Wouessidjewe, D., Ponchel, G.: Cyclodextrins and carrier systems. J. Controlled Release 62, 263–268 (1999)
Bender, M.L., Komiyama, M.: Cyclodextrin Chemistry. Springer-Verlag, Berlin Heidelberg New York, Chapter II, pp. 2–8 (1978)
Másson, M., Loftsson, T., Jónsdóttir, S., Fridriksdóttir, H., Petersen, D.S.: Stabilisation of ionic drugs through complexation with non-ionic and ionic cyclodextrins. Int. J. Pharm. 164, 45–55 (1998)
Matsui Y., Kurita T., Yagi M., Okayama T., Mochida K., Date Y.: The formation and structure of Copper(II) complexes with cyclodextrins in an alkaline solution. Bull. Chem. Soc. Jpn. 48 (7), 2187–2191 (1975)
Stoddart, J.F., Zarzycki, R.: Cyclodextrins as second-sphere ligands for transition metal complexes. Recl. Trav. Chim. Pays-Bas 107, 515–528 (1988)
Heck, R., Marsura, A.: A new symmetrically modified α-cyclodextrin tripode: selective metal complexation and fluorescence properties. Tetrahedron. Lett. 45, 281–284 (2004)
Campagna, T., Grasso, G., Rizzarelli, E., Vecchio, G.: L- and D- Cysteine derivatives of b-cyclodextrin: different molecular recognition properties of their copper(II) complexes for amino acids. Inorg. Chim. Acta 275–276, 395–400 (1988)
Rizzarelli, E., Vecchio, G.: Metal complexes of functionalized cyclodextrins as enzyme models and chiral receptors. Coord. Chem. Rev. 188, 343–364 (1999)
Burgos, A.E., Belchior, J.C., Sinisterra, R.D.: Controlled release of rhodium (II) carboxylates and their association complexes with cyclodextrins from hydroxyapatite matrix. Biomaterials 23, 2519–2526 (2002)
Reetz, M.T., Kostas, I.D., Waldvogel, S.R.: Synthesis of gold(I) complexes with a (thio)phosphine-modified β-cyclodextrin. Inorg. Chem. Comm. 5, 252–254 (2002)
De Stefano, C., Princi, P., Rigano, C., Sammartano, S.: Computer analysis of equilibrium data in solution. ESAB2M: an improved version of the ESAB program. Ann. Chim. (Rome) 77, 643–675 (1987)
De Stefano, C., Mineo, P., Rigano, C., Sammartano, S.: Ionic strength dependence of formation constants. XVII. The calculation of equilibrium concentrations and formation constants. Ann. Chim. (Rome) 83, 243–277 (1993)
Smith, R.M., Martell, A.E., Motekaitis, R.J.: NIST Critical Selected Stability Constant of Metal Complexes Databases, version 6.0 (2002)
Casale, A., Daniele, P.G., De Robertis, A., Sammartano, S.: Ionic strength dependence of formation constants. Part XI. An analysis of literature data on carboxylate ligand complexes. Ann. Chim. (Rome) 78, 249–260 (1988)
http://www.epa.gov/ttn/atw/hlthef/cadmium.html. EPA, Cadmium compounds, Hazard Summary-(April 1992, Revised in January 2000)
Acknowledgements
The financial contribution (PRIN 2004) from MIUR (Ministero dell’Istruzione, dell’Università e della Ricerca), Italy is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berto, S., Bruzzoniti, M.C., Cavalli, R. et al. Highly crosslinked ionic β-cyclodextrin polymers and their interaction with heavy metals. J Incl Phenom Macrocycl Chem 57, 637–643 (2007). https://doi.org/10.1007/s10847-006-9270-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-006-9270-3