Abstract
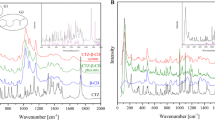
The aim of the study was to evaluate if the complexation of a hydrophilic molecule by cyclodextrins is possible. Cyclodextrins (CDs) are hydrophilic cone shaped molecules, which are used as vehicles able to include organic molecules. Because of the presence of hydroxy groups (OH) outside of the molecule, cyclodextrins are not predisposed to include hydrophilic drugs. They are therefore used to improve the solubility of poor water-soluble molecules. In order to evaluate if the complexation of a hydrophilic molecule by cyclodextrins is possible, lyophilized complexes of cysteamine hydrochloride with α-cyclodextrins (α-CD) have been realized. Six analytical techniques (High performance Liquid Chromatography coupled to UV detection, Thin-Layer Chromatography, Fourier Transform Infrared spectroscopy (FT-IR), Differential Scanning Calorimetry (DSC), Mass Spectrometry (SM) and Proton Nuclear Magnetic Resonance (NMR-NOESY spectra)) were used in order to characterize the interaction between the drug and the α-CD. The realization of complex between a cyclodextrin and a water-soluble drug seems feasible. In the case of a hydrophilic molecule, the complexation is not obtained by inclusion of the drug in the cyclodextrin, but by binding to the outside of the cone. This “external complexation” is however sufficient to improve some features of the molecule, such as organoleptic features, and to modifiy measurable parameters (FT-IR, DSC, SM and NMR-NOESY spectra).






Similar content being viewed by others
References
Saenger, W.: Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. Engl. 19, 344–362 (1980).
Rajewski, R.A., Stella, V.J.: Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J. Pharm. Sci. 85(11), 1142–1169 (1996).
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 85(10), 1017–1025 (1996).
Lach, J.L., Chin, T.F.: Inhibition of hydrolysis by means of molecular complex formation. J. Pharm. Sci. 53, 924 (1964).
Frömming, K.H., Sandman, R., Weyermann, I.: Stabilitätsprobleme bei der Verarbeitung von Einschluβverbindungen der Desoxycholsäure und des β-Cyclodextrins in Arzneiformen. Dtsch. Apoth.-Ztg, 112, 707 (1972).
Haeberlin, B., Gengenbacher, T., Meinzer, A., Fricker, G.: Cyclodextrins, useful excipients for oral peptide administration? Int. J. Pharm. 137, 103–110 (1996).
Uekama, K., Horikawa, T., Yamanaka, M., Hirayama, F.: Peracylated β-cyclodextrins as novel sustained-release carriers for a water-soluble drug, Molsidomine. J. Pharm. Pharmacol. 4, 714–717 (1994).
Andersen, F.M., Bundgaard, H., Mengel, H.B.: Formation, bioavailability and organoleptic properties of an inclusion complex of femoxetine with β-cyclodextrin. Int. J. Pharm. 21, 51–60 (1984).
Skiba, M., Fessi, H., and Puisieux, F. French Patent N° 94.02.312 (1994).
Uekama, K., Oh, K., Otagiri, M., Seo, H., Tsuruoka, M.: Improvement of some pharmaceutical properties of clofibrate by cyclodextrin complexation. Pharm. Acta Helv. 58(12), 338–342 (1983).
Miyaji, T., Inoue, Y., Acartürk, F., Imai, T., Otagiri, M., Uekama, K.: Improvement of oral bioavailability of fenbufen by cyclodextrin complexations. Acta Pharm. Nord. 4(1), 17–22 (1992).
Gal-Füzy, M., Szente, L., Szejtli, J., Harrangi, J.: Cyclodextrin stabilized volatile substances for inhalation therapy. Pharmazie, 39, 558–559 (1984).
Hou, S., Wang, P.: The preparation of inclusion compound of ranitidine - β?cyclodextrin. Chinese Pharm. J. 31(8), 479–481 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lahiani-Skiba, M., Boulet, Y., Youm, I. et al. Interaction between hydrophilic drug and α-cyclodextrins: physico-chemical aspects. J Incl Phenom Macrocycl Chem 57, 211–217 (2007). https://doi.org/10.1007/s10847-006-9194-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-006-9194-y