Abstract
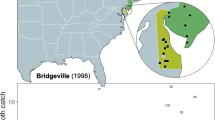
Synanthedon vespiformis L. (Lepidoptera: Sesiidae) is considered a rare insect in Sweden, discovered in 1860, with only a few observations recorded until a sex pheromone attractant became available recently. This study details a national survey conducted using pheromones as a sampling method for this species. Through pheromone trapping we captured 439 specimens in Southern Sweden at 77 sites, almost tripling the number of previously reported records for this species. The results suggest that S. vespiformis is truly a rare species with a genuinely scattered distribution, but can be locally abundant. Habitat analyses were conducted in order to test the relationship between habitat quality and the number of individuals caught. In Sweden, S. vespiformis is thought to be associated with oak hosts, but our attempts to predict its occurrence by the abundance of oaks yielded no significant relationships. We therefore suggest that sampling bias and limited knowledge on distribution may have led to the assumption that this species is primarily reliant on oaks in the northern part of its range, whereas it may in fact be polyphagous, similar to S. vespiformis found as an agricultural pest in Central and Southern Europe. We conclude that pheromones can massively enhance sampling potential for this and other rare lepidopteran species. Large-scale pheromone-based surveys provide a snapshot of true presences and absences across a considerable part of a species national distribution range, and thus for the first time provide a viable means of systematically assessing changes in distribution over time with high spatiotemporal resolution.




Similar content being viewed by others
References
Andersson K, Bergman KO, Andersson F, Hedenström E, Jansson N, Burman J, Winde I, Larsson MC, Milberg P (2014) High-accuracy sampling of saproxylic diversity indicators at regional scales with pheromones: the case of Elater ferrugineus (Coleoptera, Elateridae). Biol Conserv 171:156–166
Artportalen (2015) Swedish Species Gateway. http://www.artportalen.se/. 3 Nov 2015
Audemard H, Vigouroux A (1982) Une curieuse association parasitaire sur Pecher: Sesie (Synanthedon vespiformis) et tumeur bacterienne du collet (Agrobacterium tumefaciens). Phytoma 336:28–29
Bates CR, Scott G, Tobin M (2007) Weighing the costs and benefits of reduced sampling resolution in biomonitoring studies: perspectives from the temperate rocky intertidal. Biol Conserv 137:617–625
Bazelet CS, Samways MJ (2011) Identifying grasshopper bioindicators for habitat quality assessment of ecological networks. Ecol Indic 11:1259–1269
Bazelet CS, Samways MJ (2012) Grasshopper and butterfly local congruency in grassland remnants. J Insect Conserv 16:71–85
Bergman K-O, Jansson N, Claesson K, Palmer MW, Milberg P (2012) How much and at what scale? Multiscale analyses as decision support for conservation of saproxylic oak beetles. For Ecol Manag 265:133–141
Biggs J, Ewald N, Valentini A, Gaboriaud C, Dejean T, Griffiths RA, Foster J, Wilkinson JW, Arnell A, Brotherton P, Williams P, Dunn F (2015) Using eDNA to develop a national citizen science-based monitoring programme for the great crested newt (Triturus cristatus). Biol Conserv 183:19–28
Bommarco R, Lundin O, Smith HG, Rundlöf M (2012) Drastic historic shifts in bumble-bee community composition in Sweden. Proc Roy Soc B Biol Sci 279:309–315
Braxton SM, Raupp MJ (1995) An annotated checklist of clearwing border pests of ornamental plants trapped using commercially available pheromone lures. J Arboricult 21:177–180
Claesson K, Ek T (2009). Skyddsvärda träd i Östergötland 1997–2008. Länsstryrelsen Östergötland, 1–12
Dennis RLH, Thomas CD (2000) Bias in butterfly distribution maps: the influence of hot spots and recorder’s home range. J Insect Conserv 4:73–77
Dennis RLH, Sparks TH, Hardy PB (1999) Bias in butterfly distribution maps: the effects of sampling effort. J Insect Conserv 3:33–42
Duckworth WD, Eichlin TD (1974) Clearwing moths of Australia and New Zealand (Lepidoptera: Sesiidae). Smithsonian Institution Press, USA
Eliasson CU (2007) Synanthedon vespiformis. Fact sheet. ArtDatabanken, Swedish University of Agricultural Sciences, Uppsala
Eliasson P, Nilsson SG (2002) ‘You should hate young oaks and young noblemen’: the environmental history of oaks in eighteenth-and nineteenth-century Sweden. Environ Hist 7:659–677
Fiedler K, Schulze CH (2004) Forest modification affects diversity (but not dynamics) of speciose tropical pyraloid moth communities. Biotropica 36:615–627
Fleishman E, Murphy DD (2009) A realistic assessment of the indicator potential of butterflies and other charismatic taxonomic groups. Conserv Biol 23:1109–1116
Gärdenfors U (2010) The 2010 Red List of Swedish Species. Artdatabanken, SLU, Uppsala
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. J Insect Conserv 17:831–850
Gezon ZJ, Wyman ES, Ascher JS, Inouye DW, Irwin RE (2015) The effect of repeated, lethal sampling on wild bee abundance and diversity. Met Ecol Evol 6:1044–1054
Greatorex-Davies N, Sparks T, Woiwod I (2003) Changes in the Lepidoptera of Monks Wood NNR. Ten years of change: Woodland research at Monks Wood NNR, 90
Grove SJ (2002) Saproxylic insect ecology and the sustainable management of forests. Annu Rev Ecol Syst 33:1–23
Haniotakis GE, Koutroubas A, Sachinoglou A, Lahlou A (1999) Studies on the response of the leopard moth, Zeuzera pyrina I (Lepidoptera: Cossidae) to pheromones in apple orchards. IOBC wprs Bull 22:105–114
Hanks LM, Millar JG, Mongold-Diers JA, Wong JC, Meier LR, Reagel PF, Mitchell RF (2012) Using blends of cerambycid beetle pheromones and host plant volatiles to simultaneously attract a diversity of cerambycid species. Can J Forest Res 42:1050–1059
Harvey DJ, Hawes CJ, Gange AC, Finch P, Chesmore D, Farr I (2010) Development of non-invasive monitoring methods for larvae and adults of the stag beetle, Lucanus cervus. Insect Con Diver 4:4–14
Hauser CE, Pople AR, Possingham HP (2006) Should managed populations be monitored every year? Ecol Appl 16:807–819
Henle K, Bauch B, Auliya M, Külvik M, Pe‘er G, Schmeller DS, Framstad E (2013) Priorities for biodiversity monitoring in Europe: a review of supranational policies and a novel scheme for integrative prioritization. Ecol Indic 33:5–18
Henry PY, Lengyel S, Nowicki P, Julliard R, Clobert J, Čelik T, Gruber B, Schmeller DS, Babij V, Henle K (2008) Integrating ongoing biodiversity monitoring: potential benefits and methods. Biodivers Conserv 17:3357–3382
Holland JD, Bert DG, Fahrig L (2004) Determining the spatial scale of a species’ response to habitat. Bioscience 54:227–233
Horák J, Vodka Š, Pavlíček J, Boža P (2013) Unexpected visitors: flightless beetles in window traps. J Insect Conserv 17:441–449
Jonason D, Franzén M, Pettersson LB (2013) Transient peak in moth diversity as a response to organic farming. Basic Appl Ecol 14:515–522
Jonason D, Franzén M, Ranius T (2014) surveying moths using light traps: effects of weather and time of year. PLoS ONE 9:e92453
Kadej M, Zajac K, Ruta R, Gutowski JM, Tarnawski D, Smolis A, Olbrycht T, Malkiewicz A, Myskow E, Larsson MC, Andersson F, Hedenström E (2015) Sex pheromones as a tool to overcome the Wallacean shortfall in conservation biology: a case of Elater ferrugineus Linnaeus, 1758 (Coleoptera: Elateridae). J Insect Conserv 19:25–32
Kirby KJ, Smart SM, Black HIJ, Bunce RGH, Corney PM, Smithers RJ (2005) Long-term ecological change in British Woodlands (1971–2001): a re-survey and analysis of change based on the 103 sites in the Nature Conservancy ‘Bunce 1971’ Woodland Survey. English Nature
Larsson MC, Hedin J, Svensson GP, Tolasch T, Francke W (2003) Characteristic odor of Osmoderma eremita identified as a male-released pheromone. J Chem Ecol 29:575–587
Larsson MC, Svensson GP, Ryrholm N (2009) Monitoring rare and threatened insects with pheromone attractants. In: Samways MJ, New T, McGeoch M (eds) Insect conservation: a handbook of approaches and methods. Oxford University Press, Oxford, pp 114–116
Levi-Zada A, Ben-Yehuda S, Dunkelblum E, Gindin G, Fefer D, Protasov A, Kuznetsowa T, Manulis-Sasson S, Mendel Z (2011) Identification and field bioassays of the sex pheromone of the yellow-legged clearwing Synanthedon vespiformis (Lepidoptera: Sesiidae). Chemoecology 21:227–233
Lindenmayer DB, Gibbons P, Bourke MAX, Burgman M, Dickman CR, Ferrier S, Fitzsimons J, Freudenberger D, Garnett ST, Groves C, Hobbs RJ, Kingsford RT, Krebs C, Legge S, Lowe AJ, McLean R, Montambault J, Possingham H, Radford J, Robinson D, Smallbone L, Thomas D, Varcoe T, Vardon M, Wardle G, Woinarski J, Zerger A (2012) Improving biodiversity monitoring. Austral Ecol 37:285–294
Löfstedt C, Herrebout WM, Menken SB (1991) Sex pheromones and their potential role in the evolution of reproductive isolation in small ermine moths (Yponomeutidae). Chemoecology 2:20–28
Lovett GM, Burns DA, Driscoll CT, Jenkins JC, Mitchell MJ, Rustad L, Shanley JB, Likens GE, Haeuber R (2007) Who needs environmental monitoring? Front Ecol Environ 5:253–260
Milberg P, Bergman K-O, Johansson H, Jansson N (2014) Low host-tree preferences among saproxylic beetles: a comparison of four deciduous species. Insect Conserv Diver 7:508–522
Millar JG, McElfresh JS, Romero C, Vila M, Mari-Mena N, Lopez-Vaamonde C (2010) Identification of the sex pheromone of a protected species, the Spanish moon moth Graellsia isabellae. J Chem Ecol 36:923–932
Musa N, Andersson K, Burman J, Andersson F, Hedenström E, Jansson N, Paltto H, Westerberg L, Winde I, Larsson MC, Bergman K-O, Milberg P (2013) Using sex pheromone and a multi-scale approach to predict the distribution of a rare saproxylic beetle. PLoS ONE 8:e66149
Nilsson SG, Arup U, Baranowski R, Ekman S (1995) Tree-dependent lichens and beetles as indicators in conservation forests. Conserv Biol 9:1208–1215
Östrand F, Anderbrant O (2003) From where are insects recruited? A new model to interpret catches of attractive traps. Agric For Entomol 5:163–171
Palmqvist G (2014) Intressanta fynd av storfjärilar (Macrolepidoptera) i Sverige 2013 [Remarkable records of Macrolepidoptera in Sweden 2013]. Entomol Tidskr 135:63–76
Pellet J, Schmidt BR (2005) Monitoring distributions using call surveys: estimating site occupancy, detection probabilities and inferring absence. Biol Conserv 123:27–35
Pereira HM, Cooper DH (2006) Towards the global monitoring of biodiversity change. Trends Ecol Evol 21:123–129
Quinto J, Marcos-García MA, Brustel H, Galante E, Micó E (2013) Effectiveness of three sampling methods to survey saproxylic beetle assemblages in Mediterranean woodland. J Insect Conserv 17:765–776
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. 25 Nov 2015
Rademaekers K, Braat LC, Eichler L, Widerberg O, Jones-Walters L (2010) Costs and benefits assessment of monitoring approaches for measuring progress towards the EU 2020 biodiversity target. http://edepot.wur.nl/173781
Rosenberg DM, Danks HV, Lehmkuhl DM (1986) Importance of insects in environmental impact assessment. Environ Manag 10:773–783
Svensson GP, Liedtke C, Hedenström E, Breistein P, Bång J, Larsson MC (2012) Chemical ecology and insect conservation: optimizing pheromone-based monitoring of the threatened saproxylic click beetle Elater ferrugineus. J Insect Conserv 16:549–555
Szántóné-Veszelka M, Poós B, Szőcs G (2010) Blackberry and raspberry, new hosts of the yellow legged clearwing moth, Synanthedon vespiformis: what can the recently developed sex attractant offer in monitoring and beyond. In: IOBC working group, integrated plant protection in fruit crops subgroup “Soft Fruits”, 7th workshop on integrated soft fruit production, pp 20–23
Tobin PC, Liebhold AM, Roberts EA (2007) Comparison of methods for estimating the spread of a non-indigenous species. J Biogeogr 34:305–312
Tolasch T, Von Fragstein M, Steidle JLM (2007) Sex pheromone of Elater ferrugineus L. (Coleoptera: Elateridae). J Chem Ecol 33:2156–2166
Van der Meulen J, Groenendijk D (2005) Assessment of the mobility of day-flying moths: an ecological approach. Proc Exp Appl Entomol 16:37–50
Waring P, Townsend M (2003) Field guide to the moths of Great Britain and Ireland. British Wildlife Publishing Ltd, UK
Yamanaka T, Satoda S, Senda S, Tatsuki S (2001) Mass-trapping trials of the fall webworm, Hyphantria cunea (Drury)(Lepidoptera: Arctiidae), with synthetic sex pheromone in urban street trees. Jpn J Appl Entomol Z 12:175–183
Yoccoz NG, Nichols JD, Boulinier T (2001) Monitoring of biological diversity in space and time. Trends Ecol Evol 16:446–453
Zauli A, Chiari S, Hedenström E, Svensson GP, Carpaneto GM (2014) Using odour traps for population monitoring and dispersal analysis of the threatened saproxylic beetles Osmoderma eremita and Elater ferrugineus in central Italy. J Insect Conserv 18:801–813
Zhang Q-H, Schlyter F (1996) High recaptures and long sampling range of pheromone traps for fall web worm moth Hyphantria cunea (Lepidoptera: Arctiidae) males. J Chem Ecol 22:1783–1796
Acknowledgments
Stiftelsen Eklandskapet i Linköpings kommun, Marie-Claire Cronstedts Stiftelse, Swedish WWF, the Tranemåla Foundation, Skogssällskapet, Region Skånes miljövårdsfond, SLU Partnerskap Alnarp, and the IC-E3 Linnaeus grant (Formas, SLU) to the Division of Chemical Ecology at SLU provided grants for this project. Assistance was provided by Stefan Ekroth (expert advice, field work and species determination), Henrik Nguyen (pheromone bait and administrative support); Klas Andersson (field work); Tomas Burén and co-workers at Kalmar Municipality, Anders Jörneskog at Linköping Municipality and Kjell Antonsson at the County Administrative Board of Östergotland (various support). Thanks also go to David Heaver at Natural England for comments on the research from a national monitoring perspective. Finally, a special thanks to all landowners who gave their permission to set up traps, and their commitment to the historical legacy of their land as well as its future management.
Author’s contribution
M.C.L., L.W., P.M., N.R. & K.O.B. conceived and designed the study. J.B., M.C.L., I.W., S.O. and F.N. collected most of the data. L.W., J.B., P.M., S.O. & M.C.L. analyzed the data. J.B., M.C.L., N.R., F.N. & I.W. contributed reagents/materials/analysis tools. J.B., P.M. & M.C.L. wrote the paper with assistance from all other coauthors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burman, J., Westerberg, L., Ostrow, S. et al. Revealing hidden species distribution with pheromones: the case of Synanthedon vespiformis (Lepidoptera: Sesiidae) in Sweden. J Insect Conserv 20, 11–21 (2016). https://doi.org/10.1007/s10841-015-9835-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-015-9835-9