Abstract
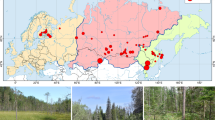
The Brazilian Atlantic Forest is a Biodiversity Hotspot, yet many biological groups in this biome are poorly known. We compiled information on the diversity of Atlantic Forest tiger moths (Arctiidae) and assessed the resemblance among localities, whether arctiid assemblages are concordant with major vegetation types, and the importance of environmental factors in structuring the variation among assemblages. Additionally, we developed a procedure composed of subsampling and procrustean analysis to assess the robustness of the results from community composition ordinations when localities differ in species richness. To do this, we built a database from specimens deposited in the ten most important Brazilian entomological collections, and mapped species richness in one-degree latitude/longitude grid cells. We employed Principal Coordinates Analysis to assess similarities among the best-sampled localities. We obtained 8,667 records including 1,193 species, representing 60 and 20% of the estimated Brazilian and Neotropical faunas, respectively. Our subsampling procedure indicated that the ordination was not greatly affected by differences in species richness, and was congruent with major vegetation types. Lowland localities on the seacoast were quite distinct in species composition. A second group included localities in montane areas in the southeast part of the biome. The last group included localities in the southern region and those with Araucaria forests, and was associated with long distances from the ocean, even distribution of precipitation throughout the year, and large annual temperature ranges.




Similar content being viewed by others
References
Axmacher JC, Brehm G, Hemp A, Tünte H, Lyaruu HVM, Müller-Hohenstein K, Fiedler K (2009) Determinants of diversity in afrotropical herbivorous insects (Lepidoptera: Geometridae): plant diversity, vegetation structure or abiotic factors? J Biogeogr 36:337–349
Baselga A (2007) Disentangling distance decay of similarity from richness gradients: response to Soininen et al. 2007. Ecography 30:838–841
Beck J, Kitching IJ (2007) Correlates of range size and dispersal ability: a comparative analysis of sphingid moths from the Indo-Australian tropics. Global Ecol. Biogeogr. 16:341–349
Biezanko CM, Freitas RG (1938) Catálogo dos insetos encontrados na cidade de Pelotas e seus arredores. I. Lepidópteros. Boletim 25. Escola de Agronomia Eliseu Maciel, Pelotas
Biezanko CM, Seta FD (1939) Catálogo dos insetos encontrados em Rio Grande e seus arredores. I. Lepidópteros. A Universal, Pelotas
Brown KS Jr, Freitas AVL (2000) Atlantic forest butterflies: indicators for landscape conservation. Biotropica 32:934–956
Brown Jr KS, Freitas AVL (1999) Lepidoptera. In: Brandão CRF, Cancello EM (eds) Biodiversidade do Estado de São Paulo: síntese do conhecimento ao final do século XX. 5. Invertebrados terrestres. Fapesp, São Paulo, pp 225–243
Canhos VP, Souza S, Giovanni R, Canhos DAL (2004) Global biodiversity informatics: setting the scene for a “new world” of ecological modeling. Biodiversity Informatics 1:1–13
Costa LP, Leite YLR, Fonseca GAB, Fonseca MT (2000) Biogeography of South American forest mammals: endemism and diversity in the Atlantic Forest. Biotropica 32:872–881
Dargie TCD (1986) Species richness and distortion in reciprocal averaging and detrended correspondence analysis. Vegetatio 65:95–98
Dennis RLH, Hardy PB (1999) Targeting squares for survey: predicting species richness and incidence of species for a butterfly atlas. Global Ecol. Biogeogr. 8:443–454
Dennis RLH, Thomas CD (2000) Bias in butterfly distribution maps: the influence of hot spots and recorder’s home range. J Insect Conserv 4:73–77
Dennis RLH, Sparks TH, Hardy PB (1999) Bias in butterfly distribution maps: the effects of sampling effort. J Insect Conserv 3:33–42
Diniz-Filho JAF, Marco Jr, PD HawkinsBA (2010) Defying the curse of ignorance: perspectives in insect macroecology and conservation biogeography. Insect Conserv. Divers. 3:172–179
Edwards JL, Lane MA, Nielsen ES (2000) Interoperability of biodiversity databases: biodiversity information on every desktop. Science 289:2312–2314
Ferreira PSF, Paula AS, Martins DS (1995) Análise faunística de Lepidoptera Arctiidae em área de reserva natural remanescente de floresta tropical em Viçosa, Minas Gerais. An. Soc. Entomol. Bras. 24:123–133
Ferro VG, Diniz IR (2008) Species biological attributes affecting the description date of tiger moths (Arctiidae) in the Brazilian Cerrado. Diversity Distrib. 14:472–482
Ferro VG, Diniz IR (2010) Riqueza e composição das mariposas Arctiidae (Lepidoptera) no Cerrado. In: Diniz I R, Marinho-Filho J, Machado R B, Cavalcanti R (eds) Cerrado: conhecimento quantitativo como subsídio para as ações de conservação. Editora Thesaurus, Brasília, pp 255–313
Ferro VG, Melo AS, Diniz IR (in press) Richness of tiger moths (Lepidoptera: Arctiidae) in the Brazilian Cerrado: how much do we know? Zoologia
Fundação SOS Mata Atlântica, INPE (2001) Atlas dos remanescentes florestais da Mata Atlântica e ecossistemas associados no período de 1995–2000. Fundação SOS Mata Atlântica and INPE, São José dos Campos
Gotelli N, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Grahan CH, Ferrier S, Huettman F, Moritz C, Peterson AT (2004) New developments in museum-based informatics and applications in biodiversity analysis. TREE 19:497–503
Guralnick RP, Hill AW, Lane M (2007) Towards a collaborative, global infrastructure for biodiversity assessment. Ecol Lett 10:663–672
Heppner JB (1991) Faunal regions and the diversity of Lepidoptera. Trop. Lepid. 2:1–85
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hilty J, Merenlender A (2000) Faunal indicator taxa selection for monitoring ecosystem health. Biol Conserv 92:185–197
Hortal J, Lobo JM, Jiménez-Valverde A (2007) Limitations of biodiversity databases: case study on seed-plant diversity in Tenerife (Canary Islands). Conserv Biol 21:853–863
Hortal J, Jiménez-Valverde A, Gómez JF, Lobo JM, Baselga A (2008) Historical bias in biodiversity inventories affects the observed realized niche of the species. Oikos 117:847–858
Hortal J, Roura-Pascual N, Sanders NJ, Rahbek C (2010) Understanding (insect) species distributions across spatial scales. Ecography 33:51–53
Instituto Brasileiro de Geografia e Estatística (2008) Mapa de Biomas. http://mapas.ibge.gov.br/biomas2/viewer.htm. Accessed 26 April 2010
Kitching RL, Orr AG, Thalib L, Mitchell H, Hopkins MS, Graham AW (2000) Moths assemblages as indicators of environmental quality in remnants of upland Australian rain forest. J Appl Ecol 37:284–297
Koleff P, Gaston KJ, Lennon JJ (2003) Measuring beta diversity for presence-absence data. J Anim Ecol 72:367–382
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam
Lewinsohn TM, Prado PI (2005) How many species are there in Brazil? Conserv Biol 19:619–624
Lobo JM (2008) Database records as a surrogate for sampling effort provide higher species richness estimations. Biodivers Conserv 17:873–881
Lobo JM, Baselga A, Hortal J, Jiménez-Valverde A, Gómez JF (2007) How does the knowledge about the spatial distribution of Iberian dung beetle species accumulate over time? Diversity Distrib. 13:772–780
Loyola RD, Kubota U, Fonseca GAB, Lewinsohn TM (2009) Key neotropical ecoregions for conservation of terrestrial vertebrates. Biodivers Conserv 18:2017–2031
Marinoni RC, Dutra RRC (1996) Levantamento da fauna entomológica do Estado do Paraná. II. Ctenuchidae (Lepidoptera). Rev. Bras. Zool. 13:435–461
Marquis RJ, Morais HC, Diniz IR (2002) Interactions among cerrado plants and their herbivores: unique or typical? In: Oliveira PS, Marquis RJ (eds) The Cerrados of Brazil: ecology and natural history of a neotropical savanna. Columbia University Press, New York, pp 306–328
McCune B (1997) Influence of noisy environmental data on canonical correspondence analysis. Ecology 78:2617–2623
Melo AS (2004) A critic of the use of jackknife and related non-parametric techniques to estimate species richness in assemblages. Comm. Ecol. 5:149–157
Moerman DE, Estabrook GF (2006) The botanist effect: counties with maximal species richness tend to be home to universities and botanists. J Biogeogr 33:1969–1974
Morrone JJ (2006) Biogeographic areas and transition zones of Latin America and the Caribbean islands based on panbiogeographic and cladistic analyses of the entomofauna. Annu Rev Entomol 51:467–494
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Oksanen J, Kindt R, Legendre P, O’Hara R, Simpson GL, Stevens MHH (2008) vegan: community ecology package. R package version 1.13-0. http://vegan.r-forge.r-project.org. Accessed 26 April 2010
Oliveira-Filho AT, Fontes MAL (2000) Patterns of floristic differentiation among Atlantic forests in southeastern Brazil and the influence of climate. Biotropica 32:793–810
Piñas Rubio FSJ, Manzano I (2003) Mariposas del Ecuador. Arctiidae, subfamilia Ctenuchinae. Volume 21b. Compañia de Jesús, Quito
Piñas Rubio FSJ, Raab-Green S, Onore G, Manzano I (2000) Mariposas del Ecuador. Family Arctiidae. Volume 20. Pontificia Universidad Católica del Ecuador, Quito
Sánchez-Fernández D, Lobo JM, Abellán P, Ribera I, Millán A (2008) Bias in freshwater biodiversity sampling: the case of Iberian water beetles. Diversity Distrib. 14:754–762
Scoble MJ (1992) The lepidoptera: form, function and diversity. Oxford University Press, New York
Soberón J, Peterson AT (2004) Biodiversity informatics: managing and applying primary biodiversity data. Phil. Trans. R. Soc. B 359:689–698
Tabarelli M, Pinto LP, Silva JMC, Hirot M (2005) Challenges and opportunities for biodiversity conservation in the Brazilian Atlantic forest. Conserv Biol 19:695–700
R Development Core Team (2009) R: A language and environment for statistical computing. R foundation for statistical computing. http://www.R-project.org. accessed 26 April 2010
Teston JA, Corseuil E (2004) Diversidade de Arctiinae (Lepidoptera, Arctiidae) capturados com armadilha luminosa, em seis comunidades no Rio Grande do Sul, Brasil. Rev. Bras. Entomol. 48:77–90
Teston JA, Specht A, Di Mare RA, Corseuil E (2006) Arctiinae (lepidoptera, arctiidae) coletados em unidades de conservação estaduais do Rio Grande do Sul. Brasil. Rev. Bras. Entomol. 50:280–286
Vasconcelos HL, Vilhena JMS, Facure KG, Albernaz ALKM (2010) Patterns of ant species diversity and turnover across 2,000 km of Amazonian floodplain forest. J Biogeogr 37:432–440
Watson A, Goodger DT (1986) Catalogue of the neotropical tiger-moths. Occasional papers on systematic entomology. No. 1
Wolda H (1981) Similarity indices, sample size and diversity. Oecologia 50:296–302
Zikán JF, Zikán W (1968) Inseto-fauna do Itatiaia e da Mantiqueira 3: lepidoptera. Pesq. Agrop. Bras. 3:45–109
Acknowledgments
We especially thank Dr. Vitor Becker for his friendly hospitality on many occasions, providing access to his moth collection and library, and training of VGF in the identification of Arctiidae. We are in debt to museum curators for allowing VGF access to the collections under their responsibility and for logistical support. We extend our gratitude to all the people who collected specimens deposited in the museums. Rafael D. Loyola and two anonymous referees offered suggestions on the manuscript. Janet W. Reid revised the English. ASM received a research fellowship (CNPq no. 302482/2008-3) and research grants (476304/2007-5, 474560/2009-0) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico and from the International Foundation for Science (IFS no. A/4107-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferro, V.G., Melo, A.S. Diversity of tiger moths in a Neotropical hotspot: determinants of species composition and identification of biogeographic units. J Insect Conserv 15, 643–651 (2011). https://doi.org/10.1007/s10841-010-9363-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-010-9363-6