Abstract
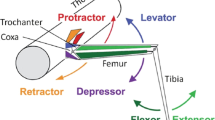
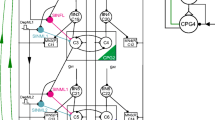
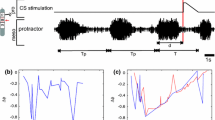
Animal locomotion requires highly coordinated working of the segmental neuronal networks that control the limb movements. Experiments have shown that sensory signals originating from the extremities play a pivotal role in controlling locomotion patterns by acting on central networks. Based on the results from stick insect locomotion, we constructed an inter-segmental model comprising local networks for all three legs, i.e. for the pro-, meso- and meta-thorax, their inter-connections and the main sensory inputs modifying their activities. In the model, the local networks are uniform, and each of them consists of a central pattern generator (CPG) providing the rhythmic oscillation for the protractor-retractor motor systems, the corresponding motoneurons (MNs), and local inhibitory interneurons (IINs) between the CPGs and the MNs. Between segments, the CPGs are connected cyclically by both excitatory and inhibitory pathways that are modulated by the aforementioned sensory inputs. Simulations done with our network model showed that it was capable of reproducing basic patterns of locomotion such as those occurring during tri- and tetrapod gaits. The model further revealed a number of elementary neuronal processes (e.g. synaptic inhibition, or changing the synaptic drive at specific neurons) that in the simulations were necessary, and in their entirety sufficient, to bring about a transition from one type of gait to another. The main result of this simulation study is that exactly the same mechanism underlies the transition between the two types of gait irrespective of the direction of the change. Moreover, the model suggests that the majority of these processes can be attributed to direct sensory influences, and changes are required only in centrally controlled synaptic drives to the CPGs.









Similar content being viewed by others
References
Borgmann, A., Hooper, S. L., & Büschges, A. (2009). Sensory feedback induced by front-leg stepping entrains the activity of central pattern generators in caudal segments of the stick insect walking system. Journal of Neuroscience, 29, 2972–2983.
Borgmann, A., Scharstein, H., & Büschges, A. (2007). Intersegmental coordination: Influence of a single walking leg on the neighboring segments in the stick insect walking system. Journal of Neurophysiology, 98, 1685–1696.
Büschges, A. (1995). Role of local nonspiking interneurons in the generation of rhythmic motor activity in the stick insect. Journal of Neurobiology, 27, 488–512.
Büschges, A. (1998). Inhibitory synaptic drive patterns motoneuronal activity in rhythmic preparations of isolated thoracic ganglia in the stick insect. Brain Research, 783, 262–271.
Büschges, A. (2005). Sensory control and organization of neural networks mediating coordination of multisegmented organs for locomotion. Journal of Neurophysiology, 93, 1127–1153.
Büschges, A., Akay, T., Gabriel, J. P., Schmidt, J. (2008). Organizing network action for locomotion: Insights from studying insect walking. Brain Research Reviews, 57, 162–171.
Büschges, A., & Gruhn, M. (2008). Mechanosensory feedback in walking: From joint control to locomotor patterns. Advances in Insect Physiology, 34, 193–230.
Büschges, A., Ludwar, B. C., Bucher, D., Schmidt, J., & DiCaprio, R. A. (2004). Synaptic drive contributing to rhythmic activation of motoneurons in the deafferented stick insect walking system. European Journal of Neuroscience, 19, 1856–1862.
Büschges, A., & Wolf, H. (1999). Phase-dependent presynaptic modulation of mechanosensory signals in the locust flight system. Journal of Neurophysiology, 81, 959–962.
Calabrese, R. L. (1995). Half-center oscillators underlying rhythmic movements. In M. Arbib (Ed.), The handbook of brain theory and neural networks (pp. 444–447). Cambridge: MIT Press.
Cohen, A. H., Holmes, P. J., & Rand, R. (1982). The nature of coupling between segmented oscillations and the lamprey spinal generator for locomotion: A mathematical model. Journal of Mathematical Biology, 13, 345–369.
Collins, J., & Richmond, S. (1994). Hard–wired central pattern generators for quadrupedal locomotion. Biological Cybernetics, 71, 375–385.
Collins, J., & Stewart, I. (1992). Hexapodal gaits and coupled nonlinear oscillator models. Biological Cybernetics, 68, 287–298.
Cruse, H. (1990). What mechanisms coordinate leg movement in walking arthropods? Trends in Neuroscience, 13, 15–21.
Cruse, H., Bartling, C., Dean, J., Kindermann, T., Schmitz, J., & Schumm, M. (2000). A simple neural network for the control of a six-legged walking system. In P. Crago, & J. Winters (Eds.), Biomechanics and neural control of posture and movement (pp. 231–239). New York: Springer.
Daun, S., Rybak, I. A., & Rubin, J. (2009). The response of a half-center oscillator to external drive depends on the intrinsic dynamics of its components: A mechanistic analysis. Journal of Computational Neuroscience, 27, 3–36.
Daun–Gruhn, S. (2010). A mathematical modeling study of inter-segmental coordination during stick insect walking. Journal of Computational Neuroscience. doi:10.1007/s10827-010-0254-3.
Delcomyn, F. (1971). The locomotion of the cockroach Periplaneta Americana. Journal of Experimental Biology, 54, 443–452.
Delcomyn, F. (1989). Walking of the American cockroach: The timing of motor activity in the legs during straight walking. Biological Cybernetics, 60, 373–384.
Dürr, V., Schmitz, J., & Cruse, H. (2004). Behavior–based modelling of hexapod locomotion: linking biology and technical application. Arthropod Structure & Development, 33, 1–13.
Ekeberg, Ö., Blümel, M., & Büschges, A. (2004). Dynamic simulation of insect walking. Arthropod Structure and Development, 33, 287–300.
El Manira, A., DiCaprio, R. A., Cattaert, D., & Clarac, F. (1991). Monosynaptic interjoint reflexes and their central modulation during fictive locomotion in crayfish. European Journal of Neuroscience, 3, 1219–1231.
Fisch, K. (2007). Untersuchungen zur Rolle und Funktion tarsaler sensorischer Signale bei der Laufmustergenerierung im Mittelbein der Stabheuschrecke Carausius morosus. (Investigations on the role and function of sensory signals of the tarsus at the generation of running patterns in the middle leg of the stick insect Carausius morosus.) Master Thesis, University of Cologne, Germany.
Gossard, J. P., Cabelguen, J. M., & Rossignol, S. (1990). Phase-dependent modulation of primary afferent depolarization in single cutaneous primary afferents evoked by peripheral stimulation during fictive locomotion in the cat. Brain Research, 537, 14–23.
Graham, D. (1972). A behavioural analysis of the temporal organisation of walking movements in the 1st instar and adult stick insect (Carausius morosus). Journal of Comparative Physiology, 81, 23–52.
Graham, D. (1977). Simulation of a model for the coordination of leg movement in free walking insects. Biological Cybernetics, 26, 187–198.
Graham, D. (1985). Pattern and control of walking in insects. Advances in Insect Physiology, 18, 31–140.
Graham, D., & Cruse, H. (1980). Coordinated walking of stick insects on a mercury surface. Journal of Experimental Biology, 92, 229–241.
Hellgren, J., Grillner, S., & Lansner, A. (1992). Computer simulation of the segmental neural network generating locomotion in lamprey by using populations of network interneurons. Biological Cybernetics, 68, 1–13.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology, 117, 500-544.
Holmes, P. J., Full, R. J., Koditschek, D., & Guckenheimer, J. (2006). The dynamics of legged locomotion: Models, analysis, and challenges. SIAM Review, 48, 207–304.
Ijspeert, A. J., Crespi, A., Ryczko, D., & Cabelguen, J. M. (2007). From swimming to walking with a salamander robot driven by a spinal cord model. Science, 315, 1416–1420.
Kittmann, R., Schmitz, J., & Büschges, A. (1996). Premotor interneurons in generation of adaptive leg reflexes and voluntary movements in stick insects. Journal of Neurobiology, 31, 512–531.
Kopell, N., Ermentrout, G. B., & Williams, T. L. (1991). On chains of oscillators forced at one end. SIAM, Journal of Applied Mathematics, 51, 1397–1417.
Laurent, G., & Burrows, M. (1989a). Distribution of intersegmental inputs to nonspiking local interneurons and motor neurons in the locust. Journal of Neuroscience, 9, 3019–3029.
Laurent, G., & Burrows, M. (1989b). Intersegmental interneurons can control the gain of reflexes in adjacent segments of the locust by their action on nonspiking local interneurons. Journal of Neuroscience, 9, 3030–3039.
Ludwar, B. C., Westmark, S., Büschges, A., & Schmidt, J. (2005). Modulation of membrane potential in mesothoracic moto- and interneurons during stick insect front leg walking. Journal of Neurophysiology, 93, 1255–1265.
Matsuoka, K. (1987). Mechanisms of frequency and pattern control in the neural rhythm generators. Biological Cybernetics, 56, 345–353.
Satterlie, R. A. (1985). Reciprocal inhibition and postinhibitory rebound produce reverberation in a locomotor pattern generator. Science, 229, 402–404.
Schilling, M., Cruse, H., & Arena, P. (2007). Hexapod walking: An expansion to walknet dealing with leg amputations and force oscillations. Biological Cybernetics, 96, 323–340.
Schmidt, J., Fischer, H., & Büschges, A. (2001). Pattern generation for walking and searching movements of a stick insect leg. II. Control of motoneuronal activity. Journal of Neurophysiology, 85, 354–361.
Schöner, G., Jiang, W. Y., & Kelso, J. A. S. (1990). A synergetic theory of quadrupedal gaits and gait transitions. Journal of Theoretical Biology, 142, 359–391.
Selverston, A. I., & Moulins, M. (1985). Oscillatory neural networks. Annual Review of Physiology, 47, 29–48.
Traub, R. D., Wong, R. K. S., Miles, R., & Michelson, H. (1991). A model of a CA3 hippocampal pyramidal neuron incorporating voltage-clamp data on intrinsic conductances. Journal of Neurophysiology, 66, 635–650.
Wadden, T., Hellgren, J., Lansner, A., & Grillner, S. (1997). Intersegmental coordination in the lamprey: Simulations using a network model without segmental boundaries. Biological Cybernetics, 76, 1–9.
Wendler, G. (1968). Ein Analogmodell der Beinbewegung eines laufenden Insekts. (An analogue model of the leg motion of a running insect.) Kybernetik, 18, 67–74.
Wendler, G. (1978). Lokomotion: Das Ergebnis zentral-peripherer Interaktion. (Locomotion: The result of central-peripheral interaction.) Verhandlungen der Deutschen Zoologischen Gesellschaft, 71, 80–96.
Westmark, S., Oliveira, E. E., & Schmidt, J. (2009). Pharmacological analysis of tonic activity in motoneurons during stick insect walking. Journal of Neurophysiology, 102, 1049–1061.
Wolf, H., & Burrows, M. (1995). Proprioceptive sensory neurons of a locust leg receive rhythmic presynaptic inhibition during walking. Journal of Neuroscience, 15, 5623–5636.
Acknowledgements
We thank the Department of Animal Physiology, University of Cologne, for providing the support and infrastructure for the Emmy Noether Research Group. We should also like to thank Drs. A. Büschges,M. Gruhn, J. Schmidt, for stimulating discussions in the course of the work, and E. Godlewska and M. Grabowska for providing experimental data on gaits.
Grant
This study was supported by Deutsche Forschungsgemeinschaft grant DA1182/1-1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Frances K. Skinner
Appendix
Appendix
1.1 Parameter values used in the CPG interneuron model and inhibitory interneuron model
1.1.1 CPG interneurons
\({\bf I}_{\textbf{NaP}}\): gNaP = 10 nS, E Na = 50 mV, V hm = − 37 mV, γ m = − 1/6 mV − 1, V hh = − 30 mV, γ h = 1/6 mV − 1, V τh = − 30 mV, γ τ = 1/12 mV − 1, ε = 0.0023; I L : g L = 2.8 nS, E L = − 65 mV; C m = 0.9154 pF; \({\bf I}_{\textbf{syn}}\): V hs = − 43 mV, γ s = − 10 mV − 1
1.1.2 Inhibitory interneurons
ϵ = 0.01 and C m = 0.21 pF, all other parameter values are the same as above.
1.1.3 Synapses and central inputs
As far as the reversal potentials of I syn and I app are concerned, their values are set in accordance with the properties of the individual synapses and central inputs. Thus E syn = 0 mV for excitatory, and E syn = − 80 mV for inhibitory synapses. The same applies to E app.
The actual values of the maximal conductances of the mutually inhibitory synapses between CPG neurons (e.g. C1–C2) are the same for all CPG neurons: g synCPG = 1.0 nS.
For the excitatory modulatory synapses, we have uniformly g syn = 0.2 nS, for the inhibitory ones: g syn = 0.1 nS. The conductance values of the central inputs I app to the CPGs are given in the text.
For all excitatory synapses on IINs from CPG neurons: g syn = 0.5 nS. All central inhibitory inputs to IINs have g d1 ≡ g d2 ≡ g app = 1.6 nS.
1.2 Details and parameter values of the motoneuron model
1.2.1 Mathematical description of the constituent currents
The general equation for the voltage-gated currents that enter the current balance equation
reads as
where x = Na,K,q,L (I syn and I app are of the same form as in the CPG neuron model: Eqs. (4) and (5)). The parameters g x and E x have the same meaning as before (cf. Section CPG neuron model). The (in)activation variables h x and m x , respectively, obey the differential equation of the form:
where y stands for h x or m x . The coefficients of Eq. (13) are nonlinear functions of the membrane potential V. They can have different forms depending on the type of the membrane current (cf. Traub et al. 1991). The parameter p is an integer.
1.2.2 Synapses and central inputs
For all inhibitory synapses on MNs from IINs: g syn = 0.2 mS/cm2.
All central excitatory inputs to MNs have g MN ≡ g app = 0.2 mS/cm2.
Rights and permissions
About this article
Cite this article
Daun–Gruhn, S., Tóth, T.I. An inter-segmental network model and its use in elucidating gait-switches in the stick insect. J Comput Neurosci 31, 43–60 (2011). https://doi.org/10.1007/s10827-010-0300-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-010-0300-1