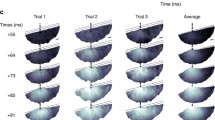
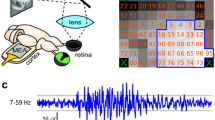
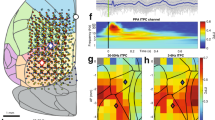
Abstract
Visual stimuli produce waves of activity that propagate across the visual cortex of fresh water turtles. This study used a large-scale model of the cortex to examine the roles of specific types of cortical neurons in controlling the formation, speed and duration of these waves. The waves were divided into three components: initial depolarizations, primary propagating waves and secondary waves. The maximal conductances of each receptor type postsynaptic to each population of neurons in the model was systematically varied and the speed of primary waves, durations of primary waves and total wave durations were measured. The analyses indicate that wave formation and speed are controlled principally by feedforward excitation and inhibition, while wave duration is controlled principally by recurrent excitation and feedback inhibition.
Similar content being viewed by others
References
Blanton MG, Shen JM, Kriegstein AR (1987) Evidence for the inhibitory neurotransmitter gamma-aminobutyric acid in a spiny and sparsely spiny nonpyramidal neurons of turtle dorsal cortex. J. Comp. Neurol. 259: 277–297.
Blanton MG, Kriegstein AR (1992) Properties of amino acid neurotransmitter receptors of embryonic cortical neurons when activated by exogenous and endogenous agonists. J. Neurophysiol. 67: 1185–1200.
Bringuier et V, Chavane F, Glaeser L, Fregnac Y (1999) Horizontal propagation of visual activity in the synaptic integration field of area 17 neurons. Science 283: 695–699.
Bower JM, Beeman D (1998) The Book of Genesis, 2nd ed. TELOS, New York.
Chagnac-Amitai Y, Connors BW (1989) Horizontal spread of synchronized activity in neocortex and its control by GABA-mediated inhibition. J. Neurophysiol. 61: 747–758.
Chervin RD, Pierce PA, Connors BW (1988) Periodicity and directionality in the propagation of epileptiform dischargers across neocortex. J. Neurophysiol. 61: 747–758.
Colombe JB, Sylvester J, Block J, Ulinski PS (2004) Subpial and stellate cells: Two populations of interneurons in turtle visual cortex. J. Comp. Neurol. 471: 333–351.
Colombe JB, Ulinski PS (1999) Temporal dispersion windows in cortical neurons. J. Comput. Neurosci. 17: 3894–3906.
Contreras D, Llinás R (2001) Voltage-sensitive dye imaging of neocortical spatiotemporal dynamics to afferent activation frequency. J. Neurosci. 21: 9403–9413.
Derdikman D, Hildesheim R, Ahissar E, Arieli A, Grinvald A (2003) Imaging spatiotemporal dynamics of surround inhibition in the barrels somatosensory cortex. J. Neurosci. 23: 3100–3105.
Du X, Ghosh BK, Ulinski PS (2005) Encoding and decoding target locations with waves in the turtle visual cortex. IEEE Trans. Biomed. Eng. 52: 566–577.
Ermentrout B (1998) The analysis of synaptically generated traveling waves. J. Comput. Neurosci. 5: 191–208.
Ghanzafar AA, Nicoleilis MAL (1999) Spatiotemporal properties of layer V neurons of the rat primary somatosensory cortex. Cerebral Cortex 9: 348–361.
Golomb D, Amitai Y (1997) Propagating neuronal discharges in neocortical slices: Computational and experimental study. J. Neurophysiol. 78: 1199–1211.
Golomb D, Ermentrout GB (2001) Bistability in pulse propagation in networks of excitatory and inhibitory populations. Phys. Rev. Lett. 86: 4179–4182.
Golomb D, Ermentrout GB (2002) Slow excitation supports propagation of slow pulses in networks of excitatory and inhibitory populations. Phys. Rev. E. 65: 061911–061916.
Grinvald A, Lieke EE, Frostig RD, Hidesheim R (1994) Cortical point spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J. Neurosci. 14: 2545–2568.
Heller SB, Ulinski PS (1987) Morphology of geniculocortical axons in turtles of the genera Pseudemys and Chrysemys. Anat. Embryol. 175: 505–515.
Kleinfeld D, Delaney KR (1996) Distributed representation of vibrissa movement in the upper layers of somatosensory cortex revealed with voltage-sensitive dyes. J. Comp. Neurol. 375: 89–108.
Larson-Prior LJ, Ulinski PS, Slater NT (1991) Excitatory amino acid receptor-mediated transmission in geniculocortical and intracortical pathways within visual cortex. J. Neurophysiol. 66: 293–306.
Mancilla JG, Fowler MH, Ulinski PS (1998) Responses of regular spiking and fast spiking cells in turtle visual cortex to light flashes. Vis. Neurosci. 15: 979–993.
Mancilla JG, Ulinski PS (2001) Role of GABAA-mediated inhibition in controlling the responses of regular spiking cells in turtle visual cortex. Vis. Neurosci. 18: 9–24.
Mulligan K, Ulinski PS (1990) Organization of the geniculocortical projection in turtles: Isoazimuth lamellae in the visual cortex. J. comp. Neurol. 296: 531–547.
Nenadic Z, Ghosh BK, Ulinski PS (2002) Modelling and estimation problems in the turtle visual cortex. IEEE Trans. Biomed. Eng. 49: 753–762.
Nenadic Z, Ghosh BK, Ulinski PS (2003) Propagating waves in visual cortex: A large-scale model of turtle visual cortex. J. Comput. Neurosci. 14: 161–184.
Petersen CCH, Sakmann B (2001) Functionally independent columns of rat somatosensory barrel cortex revealed with voltage-sensitive dye imaging. J. Neurosci. 21: 8435–8446.
Petersen CCH, Grinvald A, Sakmann B (2003) Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J. Neurosci. 23: 1298–1309.
Prechtl JC, Bullock TH, Kleinfeld D (2000) Direct evidence for local oscillatory current sources and intracortical phase gradients in turtle visual cortex. Proc. Natl. Acad. Sci. 97: 877–882.
Prechtl JC, Cohen LB, Mitra PP, Pesaran B, Kleinfeld D (1997) Visual stimuli induce waves of electrical activity in turtle visual cortex. Proc. Natl. Acad. Sci. 94: 7621–7626.
Robbins KA, Senseman DM (2004) Extracting wave structure from biological data with application to responses in turtle visual cortex. J. Comput. Neurosci. 16: 267–298.
Seidemann E, Arieli A, Grinvald A, Slovin H (2002) Dynamics of depolarization and hyperpolarization in the frontal cortex and saccade goal. Science 305: 862–865.
Senseman DM (1999) Spatiotemporal structure of depolarization spread in cortical pyramidal cell populations evoked by diffuse retinal light flashes. Vis. Neurosci. 16: 65–79.
Senseman DM, Robbins KA (2002) High-speed VSD imaging of visually evoked cortical waves: decomposition into intra- and intercortical wave motions. J. Neurophysiol. 87: 1499–1514.
Traub RD, Wong RKS, Miles R, Michelson H (1991) A model of a CA3 hippocampal pyramidal neuron incorporating voltage-clamp data on intrinsic conductances. J. Neurophysiol. 66: 635–650.
Traub RD, Jefferys JGR, Miles R, Michelson H (1993) Analysis of the propagation of disinhibition-induced afer-discharges along the guine-pig hippocampual slice in vitro. J. Physiol. (Lond.) 472: 267–287.
Ulinski PS (1986) Organization of the corticogeniculate projections in the turtle, Pseudemys scripta. J. Comp. Neurol. 254: 529–542.
Ulinski PS (1999) Neural mechanisms underlying the analysis of moving visual stimuli. In: Ulinski PS, Jones EG, Peters A, eds. Cerebral Cortex. Vol. 13. Models of Cortical Circuitry. Plenum Press, New York, pp. 283–399.
Wang W (2006) Dynamics of the turtle visual cortex and design of sensor networks. D.Sc. thesis. Washington University, Saint Louis, MO.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Campaigne, C., Ghosh, B.K. et al. Two Cortical Circuits Control Propagating Waves in Visual Cortex. J Comput Neurosci 19, 263–289 (2005). https://doi.org/10.1007/s10827-005-2288-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-005-2288-5