Abstract
Purpose
To determine if 5mM calcium chloride dihydrate supplementation of the Polyvinylpyrrolidone (PVP) media at the time of ICSI (ICSI-Ca) improves fertilization, utilization, and clinical pregnancy rates compared to ICSI alone, particularly in patients with a history of low fertilization (< 50%).
Methods
Retrospective study between 2016 and 2021 at Monash IVF Victoria on a paired cohort of patients (n = 178 patients) where an ICSI cycle was analyzed coupled with the subsequent ICSI-Ca cycle. The paired cohort was further subdivided into a low-fertilization cohort (< 50% fertilization on previous cycles: n = 66 patients) compared to the remaining patients with fertilization ≥ 50% (n = 122). Exclusion criteria included donor cycles, PGT patients, surgical sperm retrieval, women ≥ 45 years old, patients with > 6 cycles, and patients with ≤ 5 inseminated oocytes.
Results
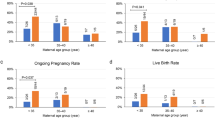
Calcium supplementation significantly increased both fertilization (28.8% ICSI vs 49.7% ICSI-Ca, P < 0.0001) and clinical pregnancy rate (4.9% ICSI vs 25.0% ICSI-Ca: P < 0.05) in the low-fertilization cohort but not in the normal-fertilization cohort. Interestingly, utilization rate significantly increased in the normal-fertilization cohort (32.6% ICSI vs ICSI-Ca: 44.9%, P < 0.01) but not in the low-fertilization cohort, although the number of embryos utilized per patient after ICSI-Ca increased in both groups.
Conclusion
Calcium supplementation does not appear to be a detrimental addition to ICSI and may improve IVF outcomes, particularly for patients with a history of low fertilization. Further investigations including prospective case-matched studies or a RCT are required to confirm these findings.



Similar content being viewed by others
References
ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine. The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod Biomed Online. 2017;35:494–510. https://doi.org/10.1016/j.rbmo.2017.06.015
Newman JE, Paul RC, Chambers GM. Assisted reproductive technology in Australia and New Zealand 2018. 2020. https://npesu.unsw.edu.au/sites/default/files/npesu/surveillances/Assisted%20Reproductive%20Technology%20in%20Australia%20and%20New%20Zealand%202018_0.pdf. Accessed 15 July 2021.
Wyns C, Bergh C, Calhaz-Jorge C, De GC, Kupka M, Motrenko T, et al. ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open. 2020;2020:1–17. https://doi.org/10.1093/hropen/hoaa032.
Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Intracytoplasmic sperm injection (ICSI) for non–male factor indications: a committee opinion. Fertil Steril. 2020;114:239–45. https://doi.org/10.1016/j.fertnstert.2020.05.032
Yanagida K, Fujikura Y, Katayose H. The present status of artificial oocyte activation in assisted reproductive technology. Reprod Med Biol. 2008;7:133–42. https://doi.org/10.1111/j.1447-0578.2008.00210.x.
Anifandis G, Michopoulos A, Daponte A, Chatzimeletiou K, Simopoulou M, Messini CI, et al. Artificial oocyte activation: physiological, pathophysiological and ethical aspects. Syst Biol Reprod Med. 2019;65:3–11. https://doi.org/10.1080/19396368.2018.1516000.
Kashir J, Nomikos M, Lai FA. Phospholipase C zeta and calcium oscillations at fertilisation: the evidence, applications, and further questions. Adv Biol Regul. 2018;67:148–62. https://doi.org/10.1016/j.jbior.2017.10.012.
Murugesu S, Saso S, Jones BP, Bracewell-Milnes T, Athanasiou T, Mania A, et al. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil Steril. 2017;108:468–82. https://doi.org/10.1016/j.fertnstert.2017.06.029.
Swann K. The role of Ca 2+ in oocyte activation during in vitro fertilization: insights into potential therapies for rescuing failed fertilization. Biochim Biophys Acta - Mol Cell Res. 2018;1865:1830–7. https://doi.org/10.1016/j.bbamcr.2018.05.003.
Rybouchkin AV, De Sutter P, Van Der Straeten F, Dhont M, Quatacker J. Fertilization and pregnancy after assisted oocyte activation and intracytoplasmic sperm injection in a case of round-headed sperm associated with deficient oocyte activation capacity. Fertil Steril. 1997;68:1144–7. https://doi.org/10.1016/s0015-0282(97)00378-6.
Kuentz P, Vanden Meerschaut F, Elinati E, Nasr-Esfahani MH, Gurgan T, Iqbal N, et al. Assisted oocyte activation overcomes fertilization failure in globozoospermic patients regardless of the DPY19L2 status. Hum Reprod. 2013;28:1054–61. https://doi.org/10.1093/humrep/det005.
Heindryckx B, De Gheselle S, Gerris J, Dhont M, De Sutter P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod Biomed Online. 2008;17:662–8. https://doi.org/10.1016/S1472-6483(10)60313-6.
Vanden Meerschaut F, Nikiforaki D, De Gheselle S, Dullaerts V, Van Den Abbeel E, Gerris J, et al. Assisted oocyte activation is not beneficial for all patients with a suspected oocyte-related activation deficiency. Hum Reprod. 2012;27:1977–84. https://doi.org/10.1093/humrep/des097.
Motteram C, Vollenhoven B, Hope N, Osianlis T, Rombauts LJ. Live birth rates after combined adjuvant therapy in IVF-ICSI cycles: a matched case-control study. Reprod Biomed Online. 2014;30:340–8. https://doi.org/10.1016/j.rbmo.2014.12.0041472-6483/Crown.
Zander-Fox D, Lam K, Pacella-Ince L, Tully C, Hamilton H, Hiraoka K, et al. PIEZO-ICSI increases fertilization rates compared with standard ICSI: a prospective cohort study. Reprod Biomed Online. 2021;43:404–12. https://doi.org/10.1016/j.rbmo.2021.05.020.
Weston G, Osianlis T, Catt J, Vollenhoven B. Blastocyst transfer does not cause a sex-ratio imbalance. Fertil Steril. 2009;92:1302–5. https://doi.org/10.1016/j.fertnstert.2008.07.1784.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8. https://doi.org/10.1016/S0015-0282(00)00518-5.
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108:393–406. https://doi.org/10.1016/j.fertnstert.2017.06.005.
Macháty Z, Funahashi H, Mayes MA, Day BN, Prather RS. Effects of injecting calcium chloride into in vitro-matured porcine oocytes. Biol Reprod. 1996;54:316–22. https://doi.org/10.1095/biolreprod54.2.316.
Fawzy M, Emad M, Mahran A, Sabry M, Fetih AN, Abdelghafar H, et al. Artificial oocyte activation with SrCl2 or calcimycin after ICSI improves clinical and embryological outcomes compared with ICSI alone: results of a randomized clinical trial. Hum Reprod. 2018;33:1636–44. https://doi.org/10.1093/humrep/dey258.
Bonte D, Ferrer-Buitrago M, Dhaenens L, Popovic M, Thys V, De Croo I, et al. Assisted oocyte activation significantly increases fertilization and pregnancy outcome in patients with low and total failed fertilization after intracytoplasmic sperm injection: a 17-year retrospective study. Fertil Steril. 2019;112:266–74. https://doi.org/10.1016/j.fertnstert.2019.04.006.
Ebner T, Montag M, Montag M, Van Der Ven K, Van Der Ven H, Ebner T, et al. Live birth after artificial oocyte activation using a ready-to-use ionophore: a prospective multicentre study. Reprod Biomed Online. 2015;30:359–65. https://doi.org/10.1016/j.rbmo.2014.11.012.
Ebner T, Oppelt P, Wober M, Staples P, Mayer RB, Sonnleitner U, et al. Treatment with Ca2+ ionophore improves embryo development and outcome in cases with previous developmental problems: a prospective multicenter study. Hum Reprod. 2015;30:97–102. https://doi.org/10.1093/humrep/deu285.
Darwish E, Magdi Y. A preliminary report of successful cleavage after calcium ionophore activation at ICSI in cases with previous arrest at the pronuclear stage. Reprod Biomed Online. 2015;31:799–804. https://doi.org/10.1016/j.rbmo.2015.08.012.
Molina LCP, Luque GM, Balestrini PA, Marín-Briggiler CI, Romarowski A, Buffone MG. Molecular basis of human sperm capacitation. Front Cell Dev Biol. 2018;6:72. https://doi.org/10.3389/fcell.2018.00072.
Águila L, Zambrano F, Arias ME, Felmer R. Sperm capacitation pretreatment positively impacts bovine intracytoplasmic sperm injection. Mol Reprod Dev. 2017;84:649–59. https://doi.org/10.1002/mrd.22834.
Morozumi K, Shikano T, Miyazaki S, Yanagimachi R. Simultaneous removal of sperm plasma membrane and acrosome before intracytoplasmic sperm injection improves oocyte activation/embryonic development. Proc Natl Acad Sci U S A. 2006;103:17661–6. https://doi.org/10.1073/pnas.0608183103.
Seita Y, Ito J, Kashiwazaki N. Removal of acrosomal membrane from sperm head improves development of rat zygotes derived from intracytoplasmic sperm injection. J Reprod Dev. 2009;55:475–9. https://doi.org/10.1262/jrd.20216.
Watanabe H, Suzuki H, Fukui Y. Fertilizability, developmental competence, and chromosomal integrity of oocytes microinjected with pre-treated spermatozoa in mice. Reproduction. 2010;139:513–21. https://doi.org/10.1530/REP-09-0270.
Visconti PE, Ning XP, Fornés MW, Alvarez JG, Stein P, Connors SA, et al. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol. 1999;214:429–43. https://doi.org/10.1006/dbio.1999.9428.
Branham MT, Mayorga LS, Tomes CN. Calcium-induced acrosomal exocytosis requires cAMP acting through a protein kinase A-independent, Epac-mediated pathway. J Biol Chem. 2006;281:8656–66. https://doi.org/10.1074/jbc.M508854200.
Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, et al. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–7. https://doi.org/10.1038/nature09769.
Alasmari W, Costello S, Correia J, Oxenham SK, Morris J, Fernandes L, et al. Ca2+ signals generated by CatSper and Ca2+ stores regulate different behaviors in human sperm. J Biol Chem. 2013;288:6248–58. https://doi.org/10.1074/jbc.M112.439356.
Luque GM, Dalotto-Moreno T, Martín-Hidalgo D, Ritagliati C, Puga Molina LC, Romarowski A, et al. Only a subpopulation of mouse sperm displays a rapid increase in intracellular calcium during capacitation. J Cell Physiol. 2018;233:9685–700. https://doi.org/10.1002/jcp.26883.
Ding D, Wang Q, Li X, Chen B, Zou W, Ji D, et al. Effects of different polyvinylpyrrolidone concentrations on intracytoplasmic sperm injection. Zygote. 2019;28:148–53. https://doi.org/10.1017/S0967199419000820.
Morbeck DE, Krisher RL, Herrick JR, Baumann NA, Matern D, Moyer T. Composition of commercial media used for human embryo culture. Fertil Steril. 2014;102:759–66. https://doi.org/10.1016/j.fertnstert.2014.05.043.
Funding
This study is funded by Monash IVF Group, Victoria.
Author information
Authors and Affiliations
Contributions
SP, FH, MG, and DZ designed the study. SP conducted all statistical analyses, interpreted results, and drafted the article. All authors (SP, FH, BV, MG, and DZ) interpreted results, reviewed the manuscript and provided substantial advice through the data analysis work, and contributed intellectually to the writing or revising of the manuscript and approval of the final version.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval for the study was obtained under Monash Health Ethics Number: 15172 M.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Popkiss, S., Horta, F., Vollenhoven, B. et al. Calcium chloride dihydrate supplementation at ICSI improves fertilization and pregnancy rates in patients with previous low fertilization: a retrospective paired treatment cycle study. J Assist Reprod Genet 39, 1055–1064 (2022). https://doi.org/10.1007/s10815-022-02407-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02407-1