Abstract
Purpose
The purpose of this study was to evaluate the capacity of bovine granulosa cells to generate reactive oxygen intermediates in response to lipopolysaccharide. We hypothesized that granulosa cells increase reactive oxygen intermediates in response to Gram-negative lipopolysaccharide in a similar manner to immune cells.
Methods
Bovine peripheral blood mononuclear cells and granulosa cells were cultured in the presence of lipopolysaccharide. Oxidative stress was evaluated using the fluorescent marker dye CellROX, and oxidative stress-related genes were measured using real-time RT-PCR.
Results
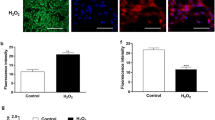
As expected, peripheral blood mononuclear cells increased oxidative stress in response to lipopolysaccharide as measured by accumulation of the fluorescent marker dye CellROX. While granulosa cells demonstrate the capacity to increase accumulation of CellROX dye in response to a positive control menadione, lipopolysaccharide had no effect on accumulation of CellROX dye. The expression of GSR, SOD1, and SOD2 were variable in peripheral blood mononuclear cells treated with lipopolysaccharide but were consistently upregulated when co-incubated with the antioxidant, N-acetyl cysteine. The expression of oxidative stressrelated genes was not altered in granulosa cells, with the exception of elevated SOD1 following lipopolysaccharide exposure in the absence of antioxidant.
Conclusions
Combined, these data suggest that while reactive stress is important in pathogen killing and inflammation in immune cells, granulosa cells do not increase oxidative stress in response to lipopolysaccharide.





Similar content being viewed by others

References
Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction. 2002;123(6):837–45.
Williams EJ, Fischer DP, Noakes DE, England GC, Rycroft A, Dobson H, et al. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology. 2007;68(4):549–59.
Williams EJ, Sibley K, Miller AN, Lane EA, Fishwick J, Nash DM, et al. The effect of Escherichia coli lipopolysaccharide and tumour necrosis factor alpha on ovarian function. Am J Reprod Immunol. 2008;60(5):462–73.
Bromfield JJ, Sheldon IM. Lipopolysaccharide initiates inflammation in bovine granulosa cells via the TLR4 pathway and perturbs oocyte meiotic progression in vitro. Endocrinology. 2011;152(12):5029–40. https://doi.org/10.1210/en.2011-1124.
Ibrahim LA, Kramer JM, Williams RS, Bromfield JJ. Human granulosa-luteal cells initiate an innate immune response to pathogen-associated molecules. Reproduction. 2016;152(4):261–70. https://doi.org/10.1530/REP-15-0573.
Price JC, Bromfield JJ, Sheldon IM. Pathogen-associated molecular patterns initiate inflammation and perturb the endocrine function of bovine granulosa cells from ovarian dominant follicles via TLR2 and TLR4 pathways. Endocrinology. 2013;154(9):3377–86. https://doi.org/10.1210/en.2013-1102.
Bromfield JJ, Santos JEP, Block J, Williams RS, Sheldon IM. Uterine infection: linking infection and innate immunity with infertility in the high-producing dairy cow. Journal of Animal Sciences. 2015;93(5):2021–33.
Herath S, Williams EJ, Lilly ST, Gilbert RO, Dobson H, Bryant CE, et al. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction. 2007;134(5):683–93.
Bromfield JJ, Sheldon IM. Lipopolysaccharide reduces the primordial follicle pool in the bovine ovarian cortex ex vivo and in the murine ovary in vivo. Biol Reprod. 2013;88(4):98. https://doi.org/10.1095/biolreprod.112.106914.
Sheldon IM, Price JC, Turner ML, Bromfield JJ, Cronin J. Uterine infection and immunity in cattle. In: Juengel JL, Miyamoto A, Price C, Reynolds LP, Smith MF, Webb R, editors. Reproduction in domestic ruminants VIII. Leciestershire: Context Products; 2014. p. 415–30.
Pelzer ES, Allan JA, Theodoropoulos C, Ross T, Beagley KW, Knox CL. Hormone-dependent bacterial growth, persistence and biofilm formation—a pilot study investigating human follicular fluid collected during IVF cycles. PLoS One. 2012;7(12):e49965. https://doi.org/10.1371/journal.pone.0049965.
Pelzer ES, Allan JA, Cunningham K, Mengersen K, Allan JM, Launchbury T, et al. Microbial colonization of follicular fluid: alterations in cytokine expression and adverse assisted reproduction technology outcomes. Hum Reprod. 2011;26(7):1799–812. https://doi.org/10.1093/humrep/der108.
Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172(4):2522–9.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801.
Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic Biol Med. 1993;15(1):69–75.
Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7(2):175–89.
Bain NT, Madan P, Betts DH. The early embryo response to intracellular reactive oxygen species is developmentally regulated. Reprod Fertil Dev. 2011;23(4):561–75. https://doi.org/10.1071/RD10148.
Agarwal A, Allamaneni SS, Nallella KP, George AT, Mascha E. Correlation of reactive oxygen species levels with the fertilization rate after in vitro fertilization: a qualified meta-analysis. Fertil Steril. 2005;84(1):228–31. https://doi.org/10.1016/j.fertnstert.2004.12.057.
Aitken RJ, Baker MA, Sawyer D. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod BioMed Online. 2003;7(1):65–70.
Xu Z, Garverick HA, Smith GW, Smith MF, Hamilton SA, Youngquist RS. Expression of follicle-stimulating hormone and luteinizing hormone receptor messenger ribonucleic acids in bovine follicles during the first follicular wave. Biol Reprod. 1995;53(4):951–7.
Price JC, Sheldon IM. Granulosa cells from emerged antral follicles of the bovine ovary initiate inflammation in response to bacterial pathogen-associated molecular patterns via Toll-like receptor pathways. Biol Reprod. 2013;89(5):119. https://doi.org/10.1095/biolreprod.113.110965.
Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141(7):2407–12.
Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264(1):182–203. https://doi.org/10.1111/imr.12266.
Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281(9):5657–67. https://doi.org/10.1074/jbc.M506172200.
Rojo AI, Salinas M, Martin D, Perona R, Cuadrado A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J Neurosci. 2004;24(33):7324–34. https://doi.org/10.1523/JNEUROSCI.2111-04.2004.
Jones PL, Ping D, Boss JM. Tumor necrosis factor alpha and interleukin-1beta regulate the murine manganese superoxide dismutase gene through a complex intronic enhancer involving C/EBP-beta and NF-kappaB. Mol Cell Biol. 1997;17(12):6970–81.
Morado SA, Cetica PD, Beconi MT, Dalvit GC. Reactive oxygen species in bovine oocyte maturation in vitro. Reprod Fertil Dev. 2009;21(4):608–14. https://doi.org/10.1071/RD08198.
Acknowledgements
The authors wish to thank Dr. John Driver for his support and valuable guidance in completing the flow cytometry analysis. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD084316.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bromfield, J.J., Iacovides, S.M. Evaluating lipopolysaccharide-induced oxidative stress in bovine granulosa cells. J Assist Reprod Genet 34, 1619–1626 (2017). https://doi.org/10.1007/s10815-017-1031-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-1031-2