Abstract
Purpose
Serum anti-Mullerian hormone (AMH) levels estimate ovarian reserve. The purpose of this study was to identify a minimum serum AMH level that correlates with acceptable clinical pregnancy rate (CPR) in women with severe diminished ovarian reserve (DOR) undergoing in vitro fertilization (IVF).
Methods(s)
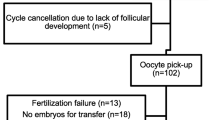
A historical cohort of severe DOR participants (age ≥35) with day 3 FSH of >10 ng/mL were included (n = 120). Participants were categorized into 3 groups: AMH <0.2 (Group 1, n = 38), AMH = 0.2-0.79 (Group 2, n = 57) and AMH ≥ 0.8 (Group 3, n = 25) ng/mL. The main outcome was CPR. The number of retrieved and mature oocytes, transferred embryos, spontaneous abortion (SAB) and live birth (LB) rates were also evaluated.
Result(s)
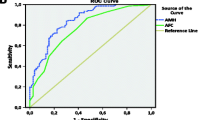
Among the three groups, there was no difference in day 3 FSH and estradiol, total gonadotropins dose used per cycle, or LB. Participants in Group 1 were two years older than those in Group 2 and had significantly higher BMI than those in Groups 2 and 3. The three groups significantly differed in AFC (Group 1< Group 2< Group 3; p = 0.001) and cycle cancellation rate (Group 1> Group 2> Group 3; p = 0.006), and had a trend toward significance in SAB rate (Group 1> Group 2> Group 3; p = 0.06). Group 3 had significantly more retrieved and mature oocytes than Groups 1 or 2. Group 2 and 3 had significantly higher CPR per cycle start compared to Group 1. Although Group 2 had significantly fewer oocytes retrieved and mature oocytes than Group 3, CPR per cycle start for both groups was not different. ROC curve indicated that the point of maximal inflection between lower and higher CPR represents an AMH value of 0.2 ng/mL.
Conclusion(s)
AMH of 0.2 ng/mL appears to be a meaningful threshold for predicting CPR in women with severe DOR at our practice. This information can be crucial during the pre-cycle counseling of these women.

Similar content being viewed by others
References
Seifer DB, Maclaughlin DT. Mullerian Inhibiting Substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88(3):539–46.
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143(3):1076–84.
Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96(8):2532–9.
Merhi Z, Minkoff H, Feldman J, Macura J, Rodriguez C, Seifer DB. Relationship of bariatric surgery to Mullerian-inhibiting substance levels. Fertil Steril. 2008;90(1):221–4.
Merhi Z. Impact of bariatric surgery on female reproduction. Fertil Steril. 2009;92(5):1501–8.
Merhi Z. Weight loss by bariatric surgery and subsequent fertility. Fertil Steril. 2007;87(2):430–2.
Merhi Z, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, et al. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: women’s interagency HIV study. Fertil Steril. 2012;98(1):228–34.
Merhi Z, Zapantis A, Berger D, Jindal S. Insufficient follicular fluid vitamin D levels are associated with anti-mullerian hormone receptor gene upregulation in human luteinized cumulus granulosa cells of small follicles. Endocr Soc 2011.
Merhi Z, Buyuk E, Israel D, Messerlian G, Eklund B, Chua S Jr.et al. Follicular fluid leptin/adiponectin ratio and age negatively correlate with anti-mullerian hormone gene expression in human luteinized mural granulosa cells. Endocr Soc 2011.
Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum anti-Mullerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab. 2012;97(7):2450–5.
Merhi Z, Buyuk E, Berger DS, Zapantis A, Israel DD, Chua Jr S, et al. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28(6):1661–9.
Buyuk E, Seifer DB, Illions E, Grazi RV, Lieman H. Elevated body mass index is associated with lower serum anti-mullerian hormone levels in infertile women with diminished ovarian reserve but not with normal ovarian reserve. Fertil Steril. 2011;95(7):2364–8.
Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77(3):468–71.
Nakhuda GS. The role of mullerian inhibiting substance in female reproduction. Curr Opin Obstet Gynecol. 2008;20(3):257–64.
Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93(3):855–64.
Gleicher N, Weghofer A, Barad DH. Anti-Mullerian hormone (AMH) defines, independent of age, low versus good live-birth chances in women with severely diminished ovarian reserve. Fertil Steril. 2010;94(7):2824–7.
Seifer DB, Baker VL, Leader B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95(2):747–50.
Buyuk E, Seifer DB, Younger J, Grazi RV, Lieman H. Random anti-Mullerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011;95(7):2369–72.
Gnoth C, Schuring AN, Friol K, Tigges J, Mallmann P, Godehardt E. Relevance of anti-Mullerian hormone measurement in a routine IVF program. Hum Reprod. 2008;23(6):1359–65.
Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril 2012; 98(6):1407–15.
Celik H, Bildircin D, Guven D, Cetinkaya MB, Alper T, Batuoglu AS. Random anti-Mullerian hormone predicts ovarian response in women with high baseline follicle-stimulating hormone levels : anti-Mullerian hormone in poor responders in assisted reproductive treatment. J Assist Reprod Genet. 2012;29(8):797–802.
Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci. 2008;1127:27–30.
Anderson KS, Segars JH. Predicting fertility with antimullerian hormone: is a cutoff value adequate? Fertil Steril. 2012;98(6):1421–2.
Barad DH, Weghofer A, Gleicher N. Utility of age-specific serum anti-Mullerian hormone concentrations. Reprod Biomed Online. 2011;22(3):284–91.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule AMH of 0.2 ng/mL appears to be a clinically meaningful cutoff for predicting a relatively “good” clinical pregnancy rate in women with severely diminished ovarian reserve at our practice.
Rights and permissions
About this article
Cite this article
Merhi, Z., Zapantis, A., Berger, D.S. et al. Determining an anti-mullerian hormone cutoff level to predict clinical pregnancy following in vitro fertilization in women with severely diminished ovarian reserve. J Assist Reprod Genet 30, 1361–1365 (2013). https://doi.org/10.1007/s10815-013-0077-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-013-0077-z