Abstract
Purpose
To investigate whether androgen conversion rates after supplementation with dehydroepiandrosterone (DHEA) differ, and whether differences between patients with diminished ovarian reserve (DOR) are predictive of pregnancy chances in association with in vitro fertilization (IVF).
Methods
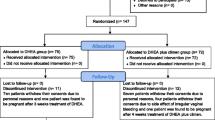
In a prospective cohort study we investigated 213 women with DOR, stratified for age (≤38 or >38 years) and ovarian FMR1 genotypes/sub-genotypes. All women were for at least 6 weeks supplemented with 75 mg of DHEA daily prior to IVF, between initial presentation and start of 1st IVF cycles. Levels of DHEA, DHEA-sulfate (DHEAS), total T (TT) and free T (FT) at baseline (BL) and IVF cycle start (CS) were then compared between conception and non-conception cycles.
Results
Mean age for the study population was 41.5 ± 4.4 years. Forty-seven IVF cycles (22.1 %) resulted in clinical pregnancy. Benefits of DHEA on pregnancy rates were statistically associated with efficiency of androgen conversion from DHEA to T and amplitude of T gain. Younger women converted significantly more efficiently than older females, and selected FMR1 genotypes/sub-genotypes converted better than others. FSH/androgen and AMH/androgen ratios represent promising new predictors of IVF pregnancy chances in women with DOR.
Conclusions
DOR at all ages appears to represent an androgen-deficient state, benefitting from androgen supplementation. Efficacy of androgen supplementation with DHEA, however, varies depending on female age and FMR1 genotype/sub-genotype. Further clarification of FMR1 effects should lead to better individualization of androgen supplementation, whether via DHEA or other androgenic compounds.





Similar content being viewed by others
Abbreviations
- AMH:
-
Anti-Müllerian hormone
- AMHR:
-
Anti-Müllerian hormone receptor
- AR:
-
Androgen receptor
- BL :
-
Baseline
- CS :
-
Cycle start
- DHEA:
-
Dehydroepiandrosterone
- DHEAS:
-
Dehydroepiandrosterone sulfate
- DOR:
-
Diminished functional ovarian reserve
- FMR1 :
-
Fragile X mental retardation 1
- FOR:
-
Functional ovarian reserve
- FSH:
-
Follicle stimulating hormone
- FSHR:
-
Follicle stimulating hormone receptor
- het :
-
Heterozygous
- IVF:
-
In vitro fertilization
- hom :
-
Homozygous
- norm :
-
Normal
- PCO:
-
Polycystic ovary
- PCOS:
-
Polycystic ovary syndrome
- POA:
-
Premature ovarian aging
- T:
-
Testosterone
- FT:
-
Free testosterone
- TT:
-
Total testosterone
- Δ:
-
Change
References
Gleicher N, Weghofer A, Barad DH. The role of androgens in follicle maturation and ovulation induction: friend or foe of infertility treatment? Reprod Biol Endocrinol. 2011;9:116.
Sen A, Hammes SR. Granulosa-cell specific androgen receptors are critical regulators of ovarian development and function. Mol Endorcinol. 2010;24:1393–403.
Barad DH, Gleicher N. Increased oocyte production after treatment with dehydroepiandrosterone. Fertil Steril. 2005;84(756):e1–3.
Mahajan DK. Polycystic ovarian disease: animal models. Endocrinol Metab Clin North Am. 1988;17:705–32.
Luchetti CG, Solano ME, Sander V, Arcos ML, Gonzales C, et al. Effects of dehydroepiandrosterone on ovarian cystogenesis and immune function. J Reprod Immunol. 2004;64:59–74.
Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve. Reprod Biol Endocrinol. 2011;9:67.
Buster JE, Casson PR, Straughn AB, Dale D, Umstot ES, et al. Postmenopausal steroid replacement with micronized dehydroepiandrosterone : preliminary oral bioavailability and dose proportionality studies Am J Obstet Gynecol. 1992;66:1163–8.
Barad DH, Brill H, Gleicher N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J Assist Reprod Genet. 2007;24:629–34.
Weghofer A, Kim A, Barad DH, Gleicher N. (2012) The impact of androgens on pregnancy potential in women with diminished ovarian reserve. Hum Reprod. In press.
Eldar-Geva T, Margalioth EJ, Gal M, Ben-Chetrit A, Algur N, et al. Serum anti-Mullerian hormone levels during controlled ovarian stimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod. 2005;20:1814–9.
Kristensen SL, Ramlau-Bansen CH, Anderson CY, Ernst E, Olsen SF, et al. The association between circulating levels of antimüllerian hormone and follicle number, androgens, and menstrual cycle characteristics in young women. Fertil Steril. 2012;97:779–85.
Frattarelli JL, Peterson EH. Effect of androgen levels on in vitro fertilization cycles. Fertil Steril. 2004;81:1713–4.
Frattarelli JL, Gerber MD. Basal and cycle androgen levels correlate with in vitro stimulation parameters but do not predict pregnancy outcome. Fertil Steril. 2006;86:51–7.
Gleicher N, Weghofer A, Barad DH. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian aging. Reprod Biomed Online. 2010;20:768–75.
Gleicher N, Weghofer A, Lee IH, Barad DH. FMR1 genotype with autoimmunity-associated polycystic ovary-like phenotype and decreased pregnancy chance. PLoS One. 2010;5:e15303.
Gleicher N, Weghofer A, Lee I, Barad DH. Association of FMR1 genotypes with in vitro fertilization (IVF) outcomes based on ethnicity/race. PLoS One. 2011;6:e18781.
Barad DH, Weghofer A, Gleicher N. Age-specific levels of basal follicle-stimulating hormone assessment of ovarian function. Obstet Gynecol. 2007;109:1404–10.
Barad DH, Weghofer A, Gleicher N. Utility of age-specific serum anti-Müllerian hormone concentrations. Reprod Biomed Online. 2011;22:284–91.
Gleicher N, Weghofer A, Barad DH. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol. 2011;9:23.
Lennie S, Smitz J. Functional Ar signaling is evident in an in vitro mouse follicle culture bioassay that encompasses most stages of folliculogenesis. Biol Reprod. 2009;80:685–95.
Gleicher N, Weghofer A, Barad DH. Improvement in diminished ovarian reserve after dehydroepiandrosterone supplementation. Reprod Biomed Online. 2010;21:360–5.
Sánchez F, Adriaenssens T, Romero S, Smitz J. Different follicle-stimulating hormone exposure regimens during antral follicle growth alter gene expression in the cumulus-oocyte complex in mice. Biol Reprod. 2010;83:514–24.
Gleicher N, Kim A, Weghofer A, Barad DH. Towards a better understanding of functional ovarian reserve: AMH (AMHo) and FSH (FSHo) hormone ratios per retrieved oocyte. J Clin Endocrinol Metabol. 2012;97:995–1004.
Gleicher N, Weghofer A, Barad DH. Anti-Müllerian hormone (AMH) defines, independent of age, low versus good live-birth chances in women with severely diminished ovarian reserve. Fertil Steril. 2010;94:2824–7.
Gleicher N, Kim A, Weghofer A, Barad DH. (2012) Differences in ovarian aging patterns between races are likely related to ovarian genotypes and sub-genotypes of the FMR1 gene. Reprod Biol Endocrinol. 2012; In press.
Yakin K, Urman B. DHEA as a miracle drug in the treatment of poor responders; hype or hope? Hum Reprod. 2011;26:1941–4.
Sunkara SK, Coomarasamy A, Arit W, Bhattacharya S. Should androgen supplementation be used for poor ovarian response in IVF? Hum Reprod. 2012;27:637–40.
Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, et al. Addition of dehydroepiandrosterone (DHEA) for poor responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum Reprod. 2010;25:2496–500.
Gleicher N, Weghofer A, Kim A, Barad DH. Comparison of ovarian FMR1 genotypes and sub-genotypes in oocyte donors and infertile women. J Assist Reprod Genet. 2012;29:529–32.
Financial disclosure
NG, AW and DHB have received research and grant support, travel funds and speaker honoraria from various pharmaceutical and medical device companies, none, however, related to here presented topics. NG and DHB are listed as inventors on two already awarded and other still pending United States patents, claiming beneficial effects on diminished ovarian reserve (DOR) and embryo ploidy from dehydroepiandrosterone (DHEA) or from other androgen supplementations. NG is owner of CHR, where this research was conducted. NG and DHB are also listed as co-inventors on a number of pending United States patents, claiming diagnostic relevance for the assessment of triple CGG repeats on the FMR1 gene in determining risk towards DOR and related issues. At time of this submission only two United States user patents with relevance to this manuscript, both DHEA-related, have been awarded (November 10, 2009; #7615544 and November 29, 2011; #8067400) The first claims benefits from supplementation of DHEA/androgens on ovarian reserve and IVF pregnancy rates in women with DOR; and the second claims decreased aneuploidy (and, therefore, miscarriage rates) and improved pregnancy rates. Among FMR1-related pending patent applications, one describes ovarian genotypes and sub-genotypes, as used in this manuscript, and claims that these genotypes and sub-genotypes reflect different ovarian aging patterns. All patent applications filed by researchers at CHR are 50 % owned by CHR and 50 % by the investigators who did the research that led to the application. A full list of all patent information can be provided on request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributions to authorship
N.G. and D.H.B. contributed equally to the manuscript. N.G. contributed to study design and data analysis, and wrote the manuscript, A.K. and D.H.B. performed data analyses and statistical analyses, A.W. contributed to study design. All authors approved the final manuscript
Capsule
This study suggests a strong statistical association between improving androgen levels and IVF pregnancy chances.
Funded by extramural funds from the Foundation for Reproductive Medicine and intramural funds from The Center for Human Reproduction (CHR) both New York, New York. Both funding organizations had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript with the following caveats: Authors, NG and DHB, are members of the Board of the Foundation for Reproductive Medicine, and author, NG, is owner of The Center for Human Reproduction (CHR), a for-profit fertility center.
Rights and permissions
About this article
Cite this article
Gleicher, N., Kim, A., Weghofer, A. et al. Starting and resulting testosterone levels after androgen supplementation determine at all ages in vitro fertilization (IVF) pregnancy rates in women with diminished ovarian reserve (DOR). J Assist Reprod Genet 30, 49–62 (2013). https://doi.org/10.1007/s10815-012-9890-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9890-z