Abstract
Purpose
To explore sperm chromosomal aneuploidy, sperm membrane and DNA integrity in infertile patients with anejaculation.
Methods
Semen samples were collected from 18 infertile men with spinal cord injury (SCI) by penile vibratory stimulation (PVS) and from 14 psychogenic anejaculation (PA) patients by percutaneous vasal sperm aspiration (PVSA). These semen samples as well as samples from 16 donors were analyzed using the hypo-osmotic swelling (HOS) test, the sperm chromatin dispersion (SCD) test and multi-color fluorescence in situ hybridization (FISH) with probes specific for chromosomes 13, 18, 21, X and Y.
Results
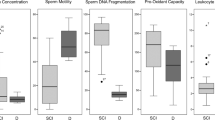
There were significant differences in the percentages of motile sperm, normal morphologic sperm and sperm DNA fragmentation between the infertile men with SCI and the control group (P < 0.05 and P < 0.01). The sperm motility was significantly greater in the PA-PVSA group than in the SCI-PVS group (P < 0.01). The number of round cells per mL of semen obtained from the 18 SCI patients by PVS was between 1 and 8 million. The rate of sperm DNA fragmentation in the SCI-PVS group was higher than that of the PA-PVSA group (P < 0.05). The aneuploidy rates for the SCI patients were 2.4-fold higher for chromosomes 13, 18 and 21 and 2.2-fold higher for chromosomes X and Y than for patients in the control group (P < 0.0001).
Conclusions
The semen quality is poorer, sperm DNA fragmentation and sperm chromosomal aneuploidies are seen at a higher rate for SCI patients compared to healthy, fertile and normospermic men. Whether the difference in yield is due to increased scrotal temperature, genitourinary infection, or other reasons requires further study.




Similar content being viewed by others
References
Chung PH, Palermo G, Schlegel PN, Veeck LL, Eid JF, Eosenwaks Z. The use of intracytoplasmic sperm injection with electroejaculates from anejaculatory men. Hum Reprod. 1998;13:1854–8.
Brackett NL. Penile vibratory stimulation for men with spinal cord injury. Hum Reprod Update. 1999;5:551–2.
McMahon CG, Abdo C, Incrocci L, Perelman M, Rowland D, Waldingger M, Xin ZC. Disorders of orgasm and ejaculation in men. J Sex Med. 2004;1:58–65.
Qiu Y, Wang SM, Yang DT, Wang LG. Percutaneous vasal sperm aspiration and intrauterine insemination for infertile males with anejaculation. Fertil Steril. 2003;79:618–20.
Denil J, Kupker W, Al-Hasani S, Schill T, Kuczyk MA, Jonas U, Diedrich K. Successful combination of transrectal electroejaculation and intracytoplasmic sperm injection in the treatment of anejaculation. Hum Reprod. 1996;11:1647–9.
Grasso M, Fortuna F, Lania C, Blanco S. Ejaculatory disorders and α1-adrenoceptor antagonists therapy: clinical and experimental researches. Transl Med. 2006;4:31.
Kafetsoulis A, Brackett NL, Ibrahim E, Attia GR, Lynne CM. Current trends in the treatment of infertility in men with spinal cord injury. Fertil Steril. 2006;86(4):781–9.
Brackett NL, Kafetsoulis A, Ibrahim E, Aballa TC, Lynne CM. Application of 2 vibrators salvages ejaculatory failures to 1 vibrator during penile vibratory stimulation in men with spinal cord injuries. J Urol. 2007;177:660–3.
Michel MC. Alpha1-adrenoceptors and ejaculatory function. Br J Pharmacol. 2007;152:289–90.
Patki P, Woodhouse J, Hamid R, Craggs M, Shah J. Effects of spinal cord injury on semen parameters. J Spinal Cord Med. 2008;31:27–32.
Soler JM, Previnaire JG, Plante P, Denys P, Chartier-Kastler E. Midodrine improves orgasm in spinal cord-injured men: the effects of autonomic stimulation. J Sex Med. 2008;5:2935–41.
Alaca R, Goktepe AS, Yildiz N, Yilmaz B, Gunduz S. Effect of penile vibratory stimulation on spasticity in men with spinal cord injury. Am J Phys Med Rehabil. 2005;84:875–9.
Hovav Y, Sibirsky O, Davarashvili A, Yaffe H. Pregnancy in a couple with a male partner born with severe bladder exstrophy. J Androl. 2004;25:960–2.
Chen SU, Shieh JY, Wang YH, Lu T, Ho HN, Yang YS. Successful pregnancy achieved by intracytoplasmic sperm injection using cryopreserved electroejaculate sperm in a couple both with spinal cord injury: a case report. Arch Phys Med Rehabil. 2005;86:1884–6.
Buch JP, Zorn BH. Evaluation and treatment of infertility in spinal cord injured men through rectal probe electroejaculation. J Urol. 1993;149:1350–4.
Ohl DA. Electroejaculation. Urol Clin North Am. 1993;20:181–8.
Chung PH, Yeko TR, Mayer JC, Sanford EJ, Maroulis GB. Assisted fertility using electroejaculation in men with spinal cord injury–a review of literature. Fertil Steril. 1995;64:1–9.
Björndahl L, Söderlund I, Kvist U. Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum Reprod. 2003;18:813–6.
World Health Organisation. Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. New York: Cambridge University Press; 1999.
World Health Organisation. WHO laboratory manual for the Examination and processing of human semen. 5th ed. WHO Press, Prepublication version; 2010, p30–32. http://whqlibdoc.who.int/publications/2010/9789241547789_eng.pdf.
Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19:1401–8.
Miciński P, Pawlicki K, Wielgus E, Bochenek M, Tworkowska I. The sperm chromatin structure assay (SCSA) as prognostic factor in IVF/ICSI program. Reprod Biol. 2009;9:65–70.
Ramos L, Wetzels AM. Low rates of DNA fragmentation in selected motile human spermatozoa assessed by the TUNEL assay. Hum Reprod. 2001;16:1703–7.
Bian Q, Xu LC, Wang SL, Xia YK, Tan LF, Chen JF, Song L, Chang HC, Wang XR. Study on the relation between occupational fenvalerate exposure and spermatozoa DNA damage of pesticide factory workers. Occup Enciron Med. 2004;61:999–1005.
Martin RH. Cytogenetic determinants of male fertility. Hum Reprod Update. 2008;14:379–90.
Fariello RM, Del Giudice PT, Spaine DM, Fraietta R, Bertolla RP, Cedenho AP. Effect of leukocytospermia and processing by discontinuous density gradient on sperm nuclear DNA fragmentation and mitochondrial activity. J Assist Reprod Genet. 2009;26:151–7.
Liu DY, Baker HW. Human sperm bound to the zona pellucida have normal nuclear chromatin as assessed by acridine orange fluorescence. Hum Reprod. 2007;22:1597–602.
Fernández JL, Muriel L, Rivero MT, et al. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66.
Fernández JL, Muriel L, Goyanes V, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84:833–16.
Zhang LH, Qiu Y, Wang KH, Wang QJ, Tao FZ, Wang LG. Measurement of sperm DNA fragmentation using bright-field microscopy: comparison between sperm chromatin dispersion test and terminal uridine nickend labeling assay. Fertil Steril. 2010;94:1027–32.
Balasuriya A, Speyer B, Serhal P, Doshi A, Harper JC. Sperm chromatin dispersion test in the assessment of DNA fragmentation and aneuploidy in human spermatozoa. Reprod Biomed Online. 2011 Mar 10.
Engh E, Clausen OP, Purvis K, Stien R. Sperm quality assessed by flow cytometry and accessory sex gland function in spinal cord injured men after repeated vibration-induced ejaculation. Paraplegia. 1993;31:3–16.
Salsabili N, Mehrsai A, Jalalizadeh B, Pourmand G, Jalaie S. Correlation of sperm nuclear chromatin condensation staining method with semen parameters and sperm functional tests in patients with spinal cord injury, varicocele, and idiopathic infertility. Urol J. 2006;3:32–7.
Brackett NL, Ibrahim E, Grotas JA, Aballa TC, Lynne CM. Higher sperm DNA damage in semen from men with spinal cord injuries compared with controls. J Androl. 2008;29:93–9.
Qiu Y, Wang SM, Yang DT, et al. Percutaneous vasal sperm aspiration and intrauterine insemination in the treatment of obstructive azoospermia. Fertil Steril. 1997;68:1135–8.
Hristova R, Ko E, Greene C, et al. Chromosome abnormalities in sperm from infertile men with aesthenoteratozoospermia. Biol Reprod. 2002;66:1781–3.
Denil J, Ohl DA, McGuire EJ, Jonas U. Treatment of anejaculation with electroejaculation. Acta Urol Belg. 1992;60:15–25.
Lin YH, Hwang JL, Tsai YL. Percutaneous epididymal sperm aspiration in psychogenic anejaculation during IVF. A report of two cases. J Reprod Med. 1999;44:894–6.
Ohl DA, Quallich SA, Sønksen J, Brackett NL, Lynne CM. Anejaculation: an electrifying approach. Semin Reprod Med. 2009;27:179–85.
Siösteen A, Forssman L, Steen Y, Sullivan L, Wickström I. Quality of semen after repeated ejaculation treatment in spinal cord injury men. Paraplegia. 1990;28:96–104.
Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–28.
Matson P, Kappelle W, Malecki I. The use of a hypo-osmotic swelling (HOS) test on sperm of the pig (Sus scrofa domesticus), emu (Dromaius novaehollandiae), Asian elephant (Elephas maximus), hamadryas baboon (Papio hamadryas hamadryas), and central rock rat (Zyzomys pedunculatus). Reprod Biol. 2009;9:181–7.
Tartagni M, Schonauer MM, Cicinelli E, Selman H, De Ziegler D, Petruzzelli F, D’Addario V. Usefulness of the hypo-osmotic swelling test in predicting pregnancy rate and outcome in couples undergoing intrauterine insemination. J Androl. 2002;23:498–502.
Check JH, Epstein R, Nowroozi K, Shanis BS, Wu CH, Bollendorf A. The hypo-osmotic swelling test as a useful adjunct to the semen analysis to predict fertility potential. Fertil Steril. 1989;52:159–61.
Chan SYW, Wang C, Chan STH, Ho PC. Differential evaluation of human sperm hypoosmotic swelling test and its relationship with the outcome of in vitro fertilization of human oocytes. Hum Reprod. 1990;5:84–8.
Milingos S, Comhaire FH, Liapi A, Aravantionos D. The value of semen characteristics and tests of sperm function in selecting couples for intra-uterine insemination. Eur J Obstet Gynecol Reprod Biol. 1996;64:115–8.
Chohan KR, Griffin JT, Lafromboise M, De Jonge CJ, Carrell DT. Comparison of chromatin assays for DNA fragmentation evaluation in human sperm. J Androl. 2006;27:53–9.
Zribi N, Feki Chakroun N, El Euch H, Ben Abdallah F, Sellami Ben Hamida A, Gargouri J, Fakhkakh F, Ammar Keskes L. Sperm DNA fragmentation and oxidation are independent of malondialdheyde. Reprod Biol Endocrinol. 2011;9:47.
Petersen CG, Vagnini LD, Mauri AL, Massaro FC, Cavagna M, Baruffi RL, Oliveira JB, Franco JG Jr. Relationship between DNA damage and sperm head birefringence. Reprod Biomed Online. 2011;21.
Yildiz C, Ottaviani P, Law N, Avearst R, Liu L, Mckerlie C. Effects of cryopreservation on sperm quality, nuclear DNA integrity, in vitro fertilization, and in vitro embryo development in the mouse. Reprod. 2007;133:585–95.
Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9:331–45.
Vegetti W, Van Assche E, Frias A, Verheyen G, Bianchi MM, Bonduelle M, Liebaers I, Van Steirteghem A. Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in-situ hybridization in infertile men. Hum Reprod. 2000;15:351–65.
Brindley GS. The fertility of men with spinal injuries. Paraplegia. 1984;22:337–48.
Halstead LS, Vervoort S, Seager SW. Rectal probe electrostimulation in the treatment of anejaculatory spinal cord injured men. Paraplegia. 1987;25:120–9.
Perkash J, Martin DE, Warner H, Blank MS, Collins DC. Reproductive biology of paraplegics: results of semen collection, testicular biopsy and serum hormone evaluation. J Urol. 1985;134:284–8.
Acknowledgements
The authors would like to thank the technical and clinical staff (Dan-tong Yang and Yan-ping Zhang) at the Key Laboratory for Improving Birth Outcome Technique. This work was supported financially by a grant from the Natural Science Foundation of Shandong Province, China (grant ZR2010HM016).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported financially by a grant from the Natural Science Foundation of Shandong Province (grant ZR2010HM016).
Capsule The rate of sperm chromosomal aneuploidy and DNA integrity for anejaculatory patients with spinal cord injury was significantly higher than healthy, fertile and normospermic men.
Rights and permissions
About this article
Cite this article
Qiu, Y., Wang, LG., Zhang, LH. et al. Sperm chromosomal aneuploidy and DNA integrity of infertile men with anejaculation. J Assist Reprod Genet 29, 185–194 (2012). https://doi.org/10.1007/s10815-011-9688-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9688-4