Abstract
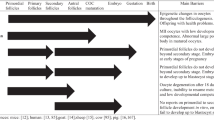
In mammalian reproduction, the oocyte depends on the ovarian follicle for most of its growth. They form a bipolar partnership and the status of one will impact the functioning of the other. When oocytes are removed from their follicle by ovulation, they have normally completed all the steps required to begin their journey into the oviduct and drive the early embryonic development. When oocytes are removed from their follicle before natural ovulation, the process by which they acquire all the important components for their journey might not be completed and their ability to mature, fertilize or develop into embryos or to term might be compromised. Animal models have been useful to define the important steps required for the oocyte’s growth phase, and in the mouse, when the oocyte has reached its full size, the program is ready. This is not the case in larger mammals where the completion of growth does not ensure that the oocyte is fully capable of undergoing all the steps to the embryo and to term. The final steps of oocyte preparation also involve a progressive condensation of the chromatin that may facilitate normal maturation but may also indirectly reduce the lifespan of the oocyte. In such a scenario, the oocyte would have an expiration date when fully competent. In humans, a number of indications may justify the aspiration of oocytes from unstimulated patients and the development of an in vitro maturation (IVM) process that would allow fertilization and subsequent development. This objective could be realized by a better understanding of the essential follicular contribution required before removing the oocyte. Therefore, this review will focus on the large animal models where IVM has been used and studied for more than 25 years. The status of the follicle at the time of oocyte recovery and the status of the oocyte’s chromatin will be described in detail as they have a significant impact on the outcome.

Similar content being viewed by others
References
Holt CE, Bullock SL. Subcellular mRNA localization in animal cells and why it matters. Science. 2009;326:1212–6.
Radford HE, Meijer HA, de Moor CH. Translational control by cytoplasmic polyadenylation in Xenopus oocytes. Biochim Biophys Acta. 2008;1779:217–29.
Memili E, Dominko T, First NL. Onset of transcription in bovine oocytes and preimplantation embryos. Mol Reprod Dev. 1998;51:36–41.
Lacham-Kaplan O, Trounson A. Reduced developmental competence of immature, in-vitro matured and postovulatory aged mouse oocytes following IVF and ICSI. Reprod Biol Endocrinol. 2008;6:58.
Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:573–85.
Pincus G. Ovum culture. Science. 1939;89:509.
Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208:349–51.
Tsafriri A, Pomerantz SH. Oocyte maturation inhibitor. Clin Endocrinol Metab. 1986;15:157–70.
Downs SM, Eppig JJ. Cyclic adenosine monophosphate and ovarian follicular fluid act synergistically to inhibit mouse oocyte maturation. Endocrinology. 1984;114:418–27.
Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–9.
Blondin P, Bousquet D, Twagiramungu H, Barnes F, Sirard MA. Manipulation of follicular development to produce developmentally competent bovine oocytes. Biol Reprod. 2002;66:38–43.
Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, et al. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994;370:68–71.
Ginther OJ, Beg MA, Donadeu FX, Bergfelt DR. Mechanism of follicle deviation in monovular farm species. Anim Reprod Sci. 2003;78:239–57.
Fair T, Hyttel P, Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev. 1995;42:437–42.
Tan JH, Wang HL, Sun XS, Liu Y, Sui HS, Zhang J. Chromatin configurations in the germinal vesicle of mammalian oocytes. Mol Hum Reprod. 2009;15:1–9.
Lodde V, Modina S, Maddox-Hyttel P, Franciosi F, Lauria A, Luciano AM. Oocyte morphology and transcriptional silencing in relation to chromatin remodeling during the final phases of bovine oocyte growth. Mol Reprod Dev. 2008;75:915–24.
Escrich L, Grau N, Meseguer M, Pellicer A, Escriba MJ. Morphologic indicators predict the stage of chromatin condensation of human germinal vesicle oocytes recovered from stimulated cycles. Fertil Steril. 2009;93:2557–64.
McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction. 2010;139:971–8.
Telfer EE, McLaughlin M. In vitro development of ovarian follicles. Semin Reprod Med. 2011;29:15–23.
Downs SM. The biochemistry of oocyte maturation. Ernst Schering Res Found Workshop 2002;41:81–99.
First NL, Leibfried-Rutledge ML, Sirard MA. Cytoplasmic control of oocyte maturation and species differences in the development of maturational competence. Prog Clin Biol Res. 1988;267:1–46.
Lonergan P, Khatir H, Carolan C, Mermillod P. Bovine blastocyst production in vitro after inhibition of oocyte meiotic resumption for 24 h. J Reprod Fertil. 1997;109:355–65.
Mayes MA, Sirard MA. Effect of type 3 and type 4 phosphodiesterase inhibitors on the maintenance of bovine oocytes in meiotic arrest. Biol Reprod. 2002;66:180–4.
Faerge I, Mayes M, Hyttel P, Sirard MA. Nuclear ultrastructure in bovine oocytes after inhibition of meiosis by chemical and biological inhibitors. Mol Reprod Dev. 2001;59:459–67.
Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod. 2010;25:2999–3011.
Sirois J, Fortune JE. Lengthening the bovine estrous cycle with low levels of exogenous progesterone: a model for studying ovarian follicular dominance. Endocrinology. 1990;127:916–25.
Sirard MA, Picard L, Dery M, Coenen K, Blondin P. The time interval between FSH administration and ovarian aspiration influences the development of cattle oocytes. Theriogenology. 1999;51:699–708.
Sirard MA, Richard F, Blondin P, Robert C. Contribution of the oocyte to embryo quality. Theriogenology. 2006;65:126–36.
Sirard MA, Parrish JJ, Ware CB, Leibfried-Rutledge ML, First NL. The culture of bovine oocytes to obtain developmentally competent embryos. Biol Reprod. 1988;39:546–52.
Chian RC, Lim JH, Tan SL. State of the art in in-vitro oocyte maturation. Curr Opin Obstet Gynecol. 2004;16:211–9.
Ali A, Sirard MA. Effect of the absence or presence of various protein supplements on further development of bovine oocytes during in vitro maturation. Biol Reprod. 2002;66:901–5.
Blondin P, Sirard MA. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol Reprod Dev. 1995;41:54–62.
Blondin P, Coenen K, Guilbault LA, Sirard MA. Superovulation can reduce the developmental competence of bovine embryos. Theriogenology. 1996;46:1191–203.
van de Leemput EE, Vos PL, Zeinstra EC, Bevers MM, van der Weijden GC, Dieleman SJ. Improved in vitro embryo development using in vivo matured oocytes from heifers superovulated with a controlled preovulatory LH surge. Theriogenology. 1999;52:335–49.
Blondin P, Dufour M, Sirard MA. Analysis of atresia in bovine follicles using different methods: flow cytometry, enzyme-linked immunosorbent assay, and classic histology. Biol Reprod. 1996;54:631–7.
Hinrichs K. The equine oocyte: factors affecting meiotic and developmental competence. Mol Reprod Dev. 2010;77:651–61.
Sirard M-A, Trounson A. Follicular factors affecting oocyte maturation and developmental competence. In: Taog RG, editor. Biology and pathology of the oocyte: its role in fertility and reproductive medicine. Cambridge: Cambridge university Press; 2003. p. 305–15.
Twagiramungu H, Guilbault LA, Proulx J, Ramkumar R, Dufour JJ. Histological populations and atresia of ovarian follicles in postpartum cattle treated with an agonist of gonadotropin-releasing hormone. J Anim Sci. 1994;72:192–200.
Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil Steril. 1991;55:109–13.
Barnes FL, Crombie A, Gardner DK, Kausche A, Lacham-Kaplan O, Suikkari AM, et al. Blastocyst development and birth after in-vitro maturation of human primary oocytes, intracytoplasmic sperm injection and assisted hatching. Hum Reprod. 1995;10:3243–7.
Cha KY, Do BR, Chi HJ, Yoon TK, Choi DH, Koo JJ, et al. Viability of human follicular oocytes collected from unstimulated ovaries and matured and fertilized in vitro. Reprod Fertil Dev. 1992;4:695–701.
Chian RC, Buckett WM, Tan SL. In-vitro maturation of human oocytes. Reprod Biomed Online. 2004;8:148–66.
Blondin P, Coenen K, Guilbault LA, Sirard MA. In vitro production of bovine embryos: developmental competence is acquired before maturation. Theriogenology. 1997;47:1061–75.
Mikkelsen AL, Smith S, Lindenberg S. Possible factors affecting the development of oocytes in in-vitro maturation. Hum Reprod. 2000;15 Suppl 5:11–7.
Fadini R, Dal Canto MB, Mignini Renzini M, Brambillasca F, Comi R, Fumagalli D, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod Biomed Online. 2009;19:343–51.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
This paper summarizes pertinent data in large mammals concerning the characteristic of the oocyte according to the follicle enclosing it and the ensuing competence to develop into an embryo.
Rights and permissions
About this article
Cite this article
Sirard, MA. Follicle environment and quality of in vitro matured oocytes. J Assist Reprod Genet 28, 483–488 (2011). https://doi.org/10.1007/s10815-011-9554-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9554-4