Abstract
Purpose:: To compare the efficiency and efficacy of two starting doses of recombinant FSH (follitropin-β, Puregon) in women undergoing IVF treatment.
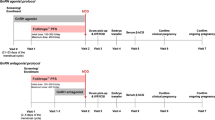
Methods: This prospective, randomized, double-blind, multicentric (N = 6) study included 192 women undergoing IVF using the long protocol of GnRH agonist who received either 100 IU or 200 IU of r-FSH per day. Gonadotropin dose adjustment was allowed after day 4 of stimulation.
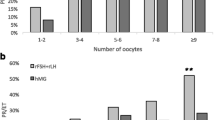
Results: The average (SD) number of oocytes retrieved was 10.9 (5.4) and 12.2 (5.6) in the 100 IU and 200 IU group respectively (p = 0.067). The total doses of Puregon administered were 1887 IU and 2559 IU in the 100 IU and 200 IU group respectively. The number of transferable embryos, and the rates of pregnancies, cancelled cycles, miscarriages and adverse events including OHSS were comparable between the two groups.
Conclusions: Women undergoing IVF have similar outcomes whether recombinant FSH is commenced in a dose of 100 IU or 200 IU for the first 4 days of stimulation.
Similar content being viewed by others
REFERENCES
Templeton A, Morris JK: Reducing the risk of multiple births by transfer of two embryos after in vitro fertilization. N Engl J Med 1998;339:573– 577
Tan SL, Maconochie N, Doyle P, Campbell S, Balen A, Bekir J, Brinsden P, Edwards RG, Jacobs HS: Cumulative conception and live-birth rates after in vitro fertilization with and without the use of long, short, and ultrashort regimens of the gonadotropin-releasing hormone agonist buserelin. Am J Obstet Gynecol 1994;171:513– 520
MacDougall MJ, Tan SL, Balen A, Jacobs HS: A controlled study comparing patients with and without polycystic ovaries undergoing in-vitro fertilization. Hum Reprod 1993;8:233– 237
Edwards RG, Lobo RA, Bouchard P: Why delay the obvious need for milder forms of ovarian stimulation? Hum Reprod 1997;12:399– 401
Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z: Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril 1989;51:651– 654
Engmann L, Sladkevicius P, Agrawal R, Bekir J, Campbell S, Tan SL: The pattern of changes in ovarian stromal and uterine artery blood flow velocities during in vitro fertilization treatment and its relationship with outcome of the cycle. Ultrasound Obstet Gynecol 1999;13:26– 33
Zaidi J, Barber J, Kyei-Mensah A, Bekir J, Campbell S, Tan SL: Relationship of ovarian stromal blood flow at the baseline ultrasound scan to subsequent follicular response in an in vitro fertilization program. Obstet Gynecol 1996;88:779– 784
Schats R, Sutter PD, Bassil S, Kremer JA, Tournaye H, Donnez J: Ovarian stimulation during assisted reproduction treatment: a comparison of recombinant and highly purified urinary human FSH. On behalf of The Feronia and Apis study group. Hum Reprod 2000;15:1691– 1697
Frydman R, Howles CM, Truong F: A double-blind, randomized study to compare recombinant human follicle stimulating hormone (FSH; Gonal-F) with highly purified urinary FSH (Metrodin) HP) in women undergoing assisted reproductive techniques including intracytoplasmic sperm injection. The French Multicentre Trialists. Hum Reprod 2000;15:520– 525
Hoomans EH, Andersen AN, Loft A, Leerentveld RA, van Kamp AA, Zech H: A prospective, randomized clinical trial comparing 150 IU recombinant follicle stimulating hormone (Puregon((R))) and 225 IU highly purified urinary follicle stimulating hormone (Metrodin-HP((R))) in a fixed-dose regimen in women undergoing ovarian stimulation. Hum Reprod 1999;14:2442– 2447
Brinsden P, Akagbosu F, Gibbons LM, Lancaster S, Gourdon D, Engrand P, Loumaye E: A comparison of the efficacy and tolerability of two recombinant human follicle-stimulating hormone preparations in patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2000;73:114– 116
Out HJ, Lindenberg S, Mikkelsen AL, Eldar-Geva T, Healy DL, Leader A, Rodriguez-Escudero FJ, Garcia-Velasco JA, Pellicer A: A prospective, randomized, double-blind clinical trial to study the efficacy and efficiency of a fixed dose of recombinant follicle stimulating hormone (Puregon) in women undergoing ovarian stimulation. Hum Reprod 1999;14:622– 627
Out HJ, David I, Ron-El R, Friedler S, Shalev E, Geslevich J, Dor J, Shulman A, Ben-Rafael Z, Fisch B, Dirnfeld M: A randomized, double-blind clinical trial using fixed daily doses of 100 or 200 IU of recombinant FSH in ICSI cycles. Hum Reprod 2001;16:1104– 1109
Out HJ, Braat DD, Lintsen BM, Gurgan T, Bukulmez O, Gokmen O, Keles G, Caballero P, Gonzalez JM, Fabregues F, Balasch J, Roulier R: Increasing the daily dose of recombinant follicle stimulating hormone (Puregon) does not compensate for the age-related decline in retrievable oocytes after ovarian stimulation. Hum Reprod 2000;15:29– 35
van Hooff MH, Alberda AT, Huisman GJ, Zeilmaker GH, Leerentveld RA: Doubling the human menopausal gonadotrophin dose in the course of an in vitro fertilization treatment cycle in low responders: A randomized study. Hum Reprod 1993;8:369– 373
Harrison RF, Jacob S, Spillane H, Mallon E, Hennelly B. A prospective randomized clinical trial of differing starter doses of recombinant follicle-stimulating hormone (follitropin-beta) for first time in vitro fertilization and intracytoplasmic sperm injection treatment cycles. Fertil Steril 2001;75:23– 31
Jones HW Jr, Out HJ, Hoomans EH, Driessen SG, Coelingh Bennink HJ: Cryopreservation: The practicalities of evaluation. Hum Reprod 1997;12:1522– 1524
Sharif K, Elgendy M, Lashen H, Afnan M: Age and basal follicle stimulating hormone as predictors of in vitro fertilisation outcome. Br J Obstet Gynaecol 1998;105:107– 112
Tinkanen H, Blauer M, Laippala P, Tuohimaa P, Kujansuu E: Prognostic factors in controlled ovarian hyperstimulation. Fertil Steril 1999;72:932– 936
Lass A, Skull J, McVeigh E, Margara R, Winston RM: Measurement of ovarian volume by transvaginal sonography before ovulation induction with human menopausal gonadotrophin for in-vitro fertilization can predict poor response. Hum Reprod 1997;12:294– 297
Ng EH, Tang OS, Ho PC: The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an IVF programme. Hum Reprod 2000;15:1937– 1942
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, S.L., Child, T.J., Cheung, A.P. et al. A Randomized, Double-Blind, Multicenter Study Comparing a Starting Dose of 100 IU or 200 IU of Recombinant Follicle Stimulating Hormone (Puregon®) in Women Undergoing Controlled Ovarian Hyperstimulation for IVF Treatment. J Assist Reprod Genet 22, 81–88 (2005). https://doi.org/10.1007/s10815-005-1497-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10815-005-1497-1