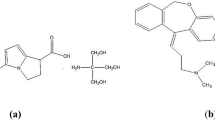
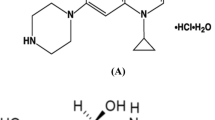
Chloramphenicol (CHL), dexamethasone sodium phosphate (DSP), and tetrahydrozoline HCl (THZ) are co-formulated for conjunctivitis treatment. The ternary mixture could not be simultaneously determined because of the overlap of the zero order absorption spectra. Herein, simple and validated UV spectrophotometric techniques have been developed for the determination of CHL, DSP, and THZ in their pure and ophthalmic dosage forms. Meanwhile, only CHL was directly determined at 284.0 nm in the range 4.0–36.0 μg/mL, while DSP and THZ were determined using single or double divisor derivative ratio spectrophotometric methods. For the single divisor derivative ratio-zero crossing spectrophotometric method (SDDR-ZC), 4.0 μg/mL CHL was used as a single divisor, where DSP and THZ were detected at 272.0 and 239.0 nm, respectively. Both DSP and THZ showed linearity ranges of 4.0–32.0 μg/mL for DSP and 3.0–24.0 μg/mL for THZ, whereas for the double divisor derivative ratio spectrophotometric method (DD-DR), (12.0 μg/mL CHL and 12.0 μg/mL THZ) and (12.0 μg/mL CHL and 12.0 μg/mL DSP) were used as double divisors for the quantitative assessment of DSP and THZ, respectively. Both DSP and THZ showed a linearity range of 4.0–32.0 μg/mL, and they were detected at 258.0 and 237.0 nm, respectively. The developed techniques were successfully applied for the determination of the three drugs in their dosage form. The proposed techniques were validated showing no significant differences when statistically compared to a reported HPLC method.
Similar content being viewed by others
References
H. Janumala, P. Kumar, and A. Baran, Ocular Pharmacol. (2012), https://doi.org/10.5772/34599.
S. Thangadurai, S. Shukla, and Y. Ananeyulut, Asian J. Chem., 13, 1456–1460 (2001).
F. Al-Rimawi, M. Kharoaf, Chromatogr. Res. Int., 1–6 (2011), https://doi.org/10.4061/2011/482308.
D. Jeffrey and J. Christopher, Ocular Pharmacology, McGraw-Hill, New York, 1707–1737 (2006)
S. N. Q. Chen, D. Zielinski, J. Chen, A. Koski, and D. Werst, J. Pharm. Biomed. Anal., 48, 732–738 (2008).
A. V. del Cuvillo, J. Sastre, J. Montoro, I. Jáuregui, I. Dávila, M. Ferrer, J. Bartra, and J. Mullol, J. Invest. Allergol. Clin. Immunol., 19, Suppl. 1, 11–18 (2009).
K. D. Tripathi, Jaypee Brothers Medical Publishers, New Delhi (1999).
S. S. Saleh, H. M. Lotfy, N. Y. Hassan, and H. Salem, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 132, 239–248 (2014), https://doi.org/10.1016/j.saa.2014.05.004.
H. M. Lotfy, S. S. Saleh, N. Y. Hassan, and H. Salem, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 126, 112–121 (2014), https://doi.org/10.1016/j.saa.2014.01.130.
B. M. Eltanany, H. R. Abd El-Hadi, H. E. Zaazaa, and M. S. Eissa, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 246, 119013 (2020), https://doi.org/10.1016/j.saa.2020.119013.
J. F. Farid, N. M. Mostafa, Y. M. Fayez, H. M. Essam, and B. M. ElTanany, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 120308, 1386–1425 (2021), https://doi.org/10.1016/j.saa.2021.120308.
H. Salem, N. Y. Hassan, H. M. Lotfy, and S. S. Saleh, J. Chromatogr. Sci., 53, 708–715 (2015), https://doi.org/10.1093/chromsci/bmu109.
M. S. Eissa, H. R. Abd El-Hadi, H. E. Zaazaa, and B. M. Eltanany, J. Planar Chromatogr., Mod. TLC (2020), https://doi.org/10.1007/s00764-020-00055-8.
H. Alaani and Y. Alnukkary, Adv. Pharm. Bull., 6, 137–141 (2016), https://doi.org/10.15171/apb.2016.020.
Y. Alnukkary, H. Alaani, Y. Alnukkary, and I. Alashkar, Int. J. Pharm. Sci. Rev. Res. (2015).
British Pharmacopoeia, Station. Off. volume II (2013).
E. Dinc, Talanta, 48, 1145–1157 (1999).
J. J. B. Nevado, C. G. Cabanillas, and F. Salinas, Talanta, 39, 547–553 (1992), https://doi.org/10.1016/0039-9140(92)80179-H.
F. Salinas, J. J. B. Nevado, and A. E. Mansilla, Talanta, 37, 347–351 (1990), https://doi.org/10.1016/0039-9140(90)80065-N.
E. Shokry, A. E. El-gendy, and M. A. Kawy, Curr. Sci. Int., 3, 352–380 (2014).
R. M. Youssef and H. M. Maher, Spectrochim. Acta A, Mol. Biomol. Spectrosc., 70, 1152–1166 (2008), https://doi.org/10.1016/j.saa.2007.10.049.
ICH, Q1A (R2). Int. Conf. on Harmonization, IFPMA, Geneva, Switzerland (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 88, No. 5, p. 821, September–October, 2021.
Rights and permissions
About this article
Cite this article
Eltanany, B.M., El-Hadi, H.R.A., Zaazaa, H.E. et al. Spectrophotometric Methods for the Determination of Chloramphenicol, Dexamethasone Sodium Phosphate, and Tetrahydrozoline HCl in their Pure and Ophthalmic Dosage Forms. J Appl Spectrosc 88, 1081–1087 (2021). https://doi.org/10.1007/s10812-021-01283-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-021-01283-4