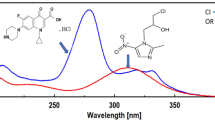
Five simple, selective, and rapid spectrophotometric methods are developed for the determination of flibanserin (FL) in the presence of its oxidative degradation products (ODPs). Stress studies are performed according to the International Conference on Harmonization (ICH) guidelines to assess the behavior of FL against oxidative, thermal, and acidic conditions. FL was stable against thermal and acidic conditions, while it was susceptible to oxidative degradation. Three of the spectrophotometric methods were univariate methods, namely, third derivative (D3), ratio difference of ratio spectra (RD), and first derivative ratio of spectra (D1R), while the other two methods were multivariate methods, namely, partial least square (PLS) and principal components regression (PCR). No preliminary separation steps were required in these methods. The chemical structures of the ODPs were confirmed using mass spectrometry and 1H NMR spectroscopy. The proposed methods were developed and validated according to the ICH guidelines. The linearity, accuracy, and precision were determined, and the selectivity was assessed by analyzing synthetic laboratory mixtures containing different ratios of FL and its ODPs. Statistical comparisons were applied to the five spectrophotometric methods, and no significant differences were found. The validated methods could be applied for routine testing and quality control.
Similar content being viewed by others
References
M. A. Sultan, A. K. Attia, and M.J. Eissa, Biomed. Chromatogr., 33, 1–9 (2019).
R. W. Invernizzi, G. Sacchetti, S. Parini, S. Acconcia, and R. Samanin, Br. J. Pharmacol., 139, 1281–1288 (2003).
A. H. Clayton, S. A. Kingsberg, and I. Goldstein, Sex. Med., 6, 59–74 (2018).
M. Géonet, P. De Sutter, and E. Zech, Sexologies, 22, e9–e15 (2013).
J. Warnock, CNS Drugs, 16, 745–753 (2006).
C. Johnson-Agbakwu, L. Brown, J. Yuan, R. Kissling, and D. J. Greenblatt, Clin. Ther., 40, 64–73 (2018).
E. D. Deeks, Drugs, 75, 1815–1822 (2015).
H. A. Croft, J. Sex. Med., 14, 1575–1584 (2017).
R. Baid and R. Agarwal, Ind. Psychiatry J., 27, 154–157 (2018).
S. Sonawane, S. Jadhav, P. Rahade, S. Chhajed, and S. Kshirsagar, Scientifica (Cairo), 2016, 1–9 (2016).
M. Blessy, R. D. Patel, P. N. Prajapati, and Y. K. Agrawal, J. Pharm. Anal., 4, 159–165 (2014).
P. N. Vaingankar and P. D. Amin, Anal. Chem. Insights, 2016, 13–20 (2016).
M. Y. Low, L. Li, X. Ge, C. L. Kee, and H. L. Koh, J. Pharm. Biomed. Anal., 57, 104–108 (2012).
M. Poplawska, A. Blazewicz, P. Zolek, and Z. Fijalek, J. Pharm. Biomed. Anal., 94, 45–53 (2014).
M. Iqbal, E. Ezzeldin, N. L. Rezk, A. A. Bajrai, and K. A. Al-Rashood, Bioanalysis, 10, 1087–1097 (2018).
P. K. Sahu, N.R. Ramisetti, T. Cecchi, S. Swain, C. S. Patro, and J. Panda, An Overview of Experimental Designs in HPLC Method Development and Validation, Elsevier B.V. (2018).
FDA Guidance for Industry: Q1A(R2) Stability Testing of New Drug Substances and Products, U.S. Department of Health and Human Services, Food and Drug Administration, 1–22 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 87, No. 5, p. 855, September–October, 2020.
Rights and permissions
About this article
Cite this article
Ahmed, R.M., Abdallah, I.A. Univariate and Chemometrics-Assisted Spectrophotometric Methods for Determination of Flibanserin in a Recently Released Dosage Form. J Appl Spectrosc 87, 976–985 (2020). https://doi.org/10.1007/s10812-020-01097-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-020-01097-w