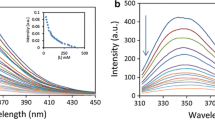
This study focuses on the interaction between methotrexate and human hemoglobin using steady-state ultraviolet-visible and fluorescence quenching methods. Fluorescence quenching was found to be valuable in assessing drug binding to hemoglobin. The quenching of methotrexate is slightly smaller than the quenching observed with related analogs (dihydrofolate and tetrahydrofolate). The quenching studies were performed at four different temperatures and various pH values. The number of binding sites for tryptophan is ~1. Parameter-dependent assays revealed that electrostatic forces play an essential role in the methotrexate–hemoglobin interaction. Furthermore, the complex was easily eluted using gel filtration chromatography.
Similar content being viewed by others
References
F. J. H. Roughton, Handbook of Physiology, Am. Physiol. Soc., Washington DC, 767–825 (1964).
D. A. Matthews, R. A. Alden, J. T. Bolin, S. T. Freer, R. Hamlin, N. Xuong, J. Kraut, M. Poe, M. Williams, and K. Hoogsteen, Science, 80, No. 197, 452–455 (1977).
M. E. Weinblatt, J. Rheumatol. Suppl., 12, 35–39 (1985).
R. Rau and G. Herborn, Clin. Exp. Rheumatol., 22, No. 35, 83–94 (2004).
E. S. L. Chan and B. N. Cronstein, Arthritis Res., 4, 266–273 (2002).
A. Saleh, M. Abuhilal, and B. Cheung, J. Turk. Acad. Dermatol., 13, 1–13 (2010).
W. Y. Leung, Hong Kong J. Dermatol. Venereol., 17, 13–19 (2009).
L. S. Frankel, Y. M. Wang, J. Shuster, R. Nitschke, E. J. Doering, and J. Pullen, J. Clin. Oncol., 1, 804–809 (1983).
R. A. Lustig, P. A. DeMare, and S. Kramer, Cancer, 37, 2703–2708 (1976).
R. L. Schilsky, B. D. Bailey, and B. A. Chabner, Proc. Natl. Acad. Sci.USA, 77, 2919–2922 (1980).
H. Breithaupt and E. Küenzlen, Oncology, 40, 85–89 (1983).
N. T. T. Tran, T.-H. Wang, C.-Y. Lin, and Y. Tai, Biochem. Eng. J., 78, 175–180 (2013).
S. Bhaskaran, C. G. Harish, and P. K. Lakshmi, J. Pharm. Res., 4, 3237–3240 (2011).
E. den Boer, R. J. W. Meesters, B. D. van Zelst, T. M. Luider, J. M. W. Hazes, S. G. Heil, and R. de Jonge, Anal. Bioanal. Chem., 405, 1673–1681 (2013).
C. Cai, X. Chen, and H. Gong, Spectrochim. Acta A: Mol. Biomol. Spectrosc., 72, 46–49 (2009).
A. Sułkowska, M. Maciążek-Jurczyk, B. Bojko, J. Równicka, and W. W. Sułkowski, J. Mol. Struct., 891, 278–283 (2008).
F. Ding, Y. Sun, J.-X. Diao, X.-N. Li, X.-L. Yang, Y. Sun, and L. Zhang, J. Photochem. Photobiol. B, 106, 53–60 (2012).
B. Sengupta, S. Chakraborty, M. Crawford, J. M. Taylor, L. E. Blackmon, P. K. Biswas, and W. H. Kramer, Int. J. Biol. Macromol., 51, 250–258 (2012).
J. Xi and R. Guo, Int. J. Biol. Macromol., 40, 305–311 (2007).
P. Mandal, M. Bardhan, and T. Ganguly, J. Photochem. Photobiol. B., 99, 78–86 (2010).
A. J. Martino and F. A. Ferrone, Biophys. J., 56, 781–794 (1989).
J. Tang, C. Yang, L. Zhou, F. Ma, S. Liu, S. Wei, J. Zhou, and Y. Zhou, Spectrochim. Acta. A. Mol. Biomol. Spectrosc., 96, 461–467 (2012).
S. V. Lepeshkevich and B. M. Dzhagarov, FEBS J., 272, 6109–6119 (2005).
S. V. Lepeshkevich, N. V. Konovalova, I. I. Stepuro, and B. M. Dzhagarov, J. Mol. Struct., 735-736, 307–313 (2005).
M. K. Safo, M. H. Ahmed, M. S. Ghatge, and T. Boyiri, Biochim. Biophys. Acta, 1814, 797–809 (2011).
Y. Q. Wang, H. M. Zhang, and Q. H. Zhou, Eur. J. Med. Chem., 44, 2100–2105 (2009).
Y. Q. Wang, H. M. Zhang, G. C. Zhang, S. X. Liu, Q. H. Zhou, Z. H. Fei, and Z. T. Liu, Int. J. Biol. Macromol., 41, 243–250 (2007).
Z. Chi, R. Liu, B. Yang, and H. Zhang, J. Hazard. Mater., 180, 741–747 (2010).
F. Ding, W. Liu, Y. Sun, X.-L. Yang, Y. Sun, and L. Zhang, J. Mol. Struct., 1007, 81–87 (2012).
X. Yan, B. Liu, B. Chong, and S. Cao, J. Lumin., 142, 155–162 (2013).
H. Cheng, H. Liu, W. Bao, and G. Zou, J. Photochem. Photobiol. B., 105, 126–132 (2011).
S. Maitra, B. Saha, C. R. Santra, A. Mukherjee, S. Goswami, P. K. Chanda, and P. Karmakar, Int. J. Biol. Macromol., 41, 23–29 (2007).
S. Tunç, A. Cetinkaya, and O. Duman, J. Photochem. Photobiol. B, 120, 59–65 (2013).
S. S. Lehrer, Biochemistry, 10, 3254–3263 (1971).
J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd ed., Springer, New York (2006).
K. Chen, S. K. Ballas, R. R. Hantgan, and D. B. Kim-Shapiro, Biophys. J., 87, 4113–4121 (2004).
M. Coppey, D. M. Jameson, and B. Alpert, FEBS Lett., 126, 191–194 (1981).
J. Albani, Structure and Dynamics of Macromolecules: Absorption and Fluorescence Studies, 1st ed., Elsevier, Amsterdam (2004), pp. 345–372.
G. M. Artmann, L. Burns, J. M. Canaves, A. Temiz-Artmann, G. W. Schmid-Schönbein, S. Chien, and C. Maggakis-Kelemen, Eur. Biophys. J., 33, 490–496 (2004).
M. Poe, J. Biol. Chem., 252, 3724–3728 (1977).
S. Y. Park, T. Yokoyama, N. Shibayama, Y. Shiro, and J. R. H. Tame, J. Mol. Biol., 360, 690–701 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 82, No. 2, pp. 284–291, March–April, 2015.
Rights and permissions
About this article
Cite this article
Zaharia, M., Gradinaru, R. Interaction of Human Hemoglobin with Methotrexate. J Appl Spectrosc 82, 278–285 (2015). https://doi.org/10.1007/s10812-015-0098-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-015-0098-8