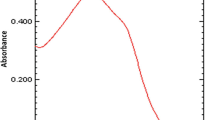
A sensitive, precise, and cost-effective UV-spectrophotometric method is described for the determination of pheniramine maleate (PAM) in bulk drug and tablets. The method is based on the measurement of absorbance of a PAM solution in 0.1 N HCl at 264 nm. As per the International Conference on Harmonization (ICH) guidelines, the method was validated for linearity, accuracy, precision, limits of detection (LOD) and quantification (LOQ), and robustness and ruggedness. A linear relationship between absorbance and concentration of PAM in the range of 2–40 μg/ml with a correlation coefficient (r) of 0.9998 was obtained. The LOD and LOQ values were found to be 0.18 and 0.39 μg/ml PAM, respectively. The precision of the method was satisfactory: the value of relative standard deviation (RSD) did not exceed 3.47%. The proposed method was applied successfully to the determination of PAM in tablets with good accuracy and precision. Percentages of the label claims ranged from 101.8 to 102.01% with the standard deviation (SD) from 0.64 to 0.72%. The accuracy of the method was further ascertained by recovery studies via a standard addition procedure. In addition, the forced degradation of PAM was conducted in accordance with the ICH guidelines. Acidic and basic hydrolysis, thermal stress, peroxide, and photolytic degradation were used to assess the stability-indicating power of the method. A substantial degradation was observed during oxidative and alkaline degradations. No degradation was observed under other stress conditions.
Similar content being viewed by others
References
K. D. Thripathi, 2008 Histamine and Antihistamines in Essentials of Medical Pharmacology, Jaypee Brothers’ Medical Publishers, 6th ed., 155 (2008).
British Pharmacopoeia, the Stationary Office, London, Vol. I & II (2009).
United States Pharmacopoeia, 30, NF 25, 2917 (2007).
J. Martens, J. Chromatogr., 673, 183 (1995).
http://dx.doi.org/10.1039/an9871200867Y. M. El-Sayed, E. M. http://dx.doi.org/10.1039/an9871200867El-Sayed, S. H. Niazy, J. Liq. Chromatogr. Rel. Technol., 18, 763 (1995).
S. El-Gezawi, N. Omar, N. El-Rabbat, and J. H. Perrin, J. Pharm. Biomed. Anal., 6, 393 (1988).
S. M. El-Gizawy and A. N. Ahmed, The Analyst, 112, 867 (1987).
D. R. Heidemann, J. Pharm. Sci., 70, 820 (1981).
A. G. Ghanekar and V. D. Gupta. Am. J. Hosp. Phar., 34, 651 (1977).
R. P. Rosario, S. El-Gizawy, J. H. Perrin, and C. M. Riley, Drug Dev. and Ind. Pharm., 12, 2443 (1986).
S. K. Pant, B. K. Maitin, and C. L. Jain, Indian Drugs., 28, 105 (1990).
V. D. Gupta and A. G. Ghanekar, J. Pharm. Sci., 66, 895(1977).
S. P. Subramaniyan and S. K. Das, J. AOAC Int., 87, 1319 (2004)
Y. Chang, C. Lee, T. Shyu, and C. Chuang, Chin. Electron. Period. Serv., 73 (1987).
H. L. Wu, C. H. Huang, S. H. Chen, and S. M. Wu, J. Chromatogr. Sci., 37 (1999).
P. Mikus, I. Valakova, and E. Havrane, J. Pharm. Biomed. Anal., 38, 442 (2005).
K. Nikolic, R. Popovic, and M.Medenica, Arh. Farm., 29, 109 (1979).
F. A. Mohamed, A. M. I. Mohamed, H. A. Mohamed, and S. A. Hussein, Talanta, 43, 1931(1996).
S. Abdel Fattah, K. O. Kelany, B. A. El-Zeany, and M. F. El-Tarras, Anal. Lett., 20, 1667 (1987).
C. A. Lucy and F. F. Cantwell, Anal. Chem., 58, 2727 (1986).
ICH-Q1A, Stability Testing of New Drug Substances and Products, International Conference on Harmonisation, Geneva, February 2003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 79, No. 1, pp. 141–149, January–February, 2012.
Rights and permissions
About this article
Cite this article
Raghu, M.S., Basavaiah, K., Ramesh, P.J. et al. Development and validation of a UV-spectrophotometric method for the determination of pheniramine maleate and its stability studies. J Appl Spectrosc 79, 131–138 (2012). https://doi.org/10.1007/s10812-012-9574-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-012-9574-6