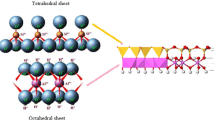
A method to modify a montmorillonite (MMT) clay mineral (CM) surface by surfactant (SA) cations with simultaneous doping by multiwall carbon nanotubes (MWNT) has been proposed. The structure and spectroscopic properties of composites based on MMT from two deposits (Cherkassy and Pyzhevsk, Ukraine) that differ in the inorganic impurity contents and cation-exchange capacities (CEC) have been investigated. Cetyltrimethylammonium bromide (CTAB) was used as the SA. According to x-ray diffraction analysis, CTA+ cations intercalated into MMT interplanar spaces expand them significantly whereas MWNTs do not affect the MMT galleries due to the much larger sizes of the former. Studies of the composite materials by IR spectroscopy revealed the mutual influence of the components appearing as the ordering of near-surface layers in the aluminosilicate framework and a change in the modifier methylene chain conformation at the interphase boundary. The majority of CTAB (~90%) is shown to be located inside the MMT galleries, the packing arrangement of which depends on the CEC value and affects the interplanar distances in MMT. The alkyl chains of the CTA+ cations on the outer surface of the MMT plates are sorbed by nanotubes, thus providing contact between the organoclay and MWNT surfaces.
Similar content being viewed by others
References
A. Dawid and Z. Gburski, J. Non-Cryst. Solids, 353, 4339–4343 (2007).
N. Lebovka, T. Dadakova, L. Lysetskiy, O. Melezhyk, G. Puchkovska, T. Gavrilko, J. Baran, and M. Drozd, J. Mol. Struct., 889, 135–143 (2008).
N. Lebovka, A. Goncharuk, V. Melnik, and G. Puchkovska, Physica, E, 41, 1554–1560 (2009).
R. Rastogi, R. Kaushal, S, K. Tripathi, A. L. Sharma, I. Kaur, and L. M. Bharadwaj, J. Colloid Interface Sci., 328, 421–428 (2008).
M. Lisunova, N. Lebovka, O. Melezhyk, and Yu. Boyko, J. Colloid Interface Sci., 299, 740–746 (2006).
S. Peeterbroeck, M. Alexandre, J. B. Nagy, C. Pirlot, A. Fonseca, N. Moreau, G. Philippin, J. Deelhalle, Z. Mekhalif, R. Sponken, G. Beyer, and P. Duboes, Compos. Sci. Technol., 64, 2317–2323 (2004).
J. P. Zhang and A. Q. Wang, Express Polymer Lett., 3, No. 5, 302–308 (2009).
B. H. Cho, I. R. Hwang, Y.-S. Lee, J. M. Jeong, K. J. Son, and C. Nan, J. Nanosci. Nanotechnol., 8, No. 10, 5516–5520 (2008).
S. Bourbigot, S. Duquesne, G .Fontaine, S. Bellayer, T. Turf, and F. Samyn, Mol. Cryst. Liq. Cryst., 486, 325/[1367]–339/[1381] (2008).
S. Bourbigot, S. Duquesne, and C. Jama, Macromol. Symposia, 233, 180–190 (2006).
P. Dubais and M. Alexandre, Adv. Eng. Mater., 8, No. 3, 147–154 (2006).
G. Beyer, Fire Mater., 29, No. 2, 61–69 (2005).
D. Gournis, M. A. Karakassides, T. Bakas, N. Boukos, and D. Petridis, Carbon, 40, 2641–2646 (2002).
X. Du, Z. Jiang, X. Meng, Z. Wang, H. Yu, M. Li, and T. Tang, J. Phys. Chem. C, 112, No. 17, 6638–6642 (2008).
L. Stobinski, J. Mazurkiewicz, P. Tomasik, J. Peszke, and H. M. Lin, Mater. Sci. Poland, 25, No. 3, 679–686 (2007).
C. Wei, Phys. Rev. B: Condens. Matter Mater. Phys., 80, 085409 (1–7) (2009).
E. G. Kukovskii, Structural Features and Physicochemical Properties of Clay Minerals [in Russian], Naukova Dumka, Kiev (1966); pp. 3–10, 51–53, 78.
J. Zhu, H. He, L. Zhu, X. Wen, and F. Deng, J. Colloid Interface Sci., 286, 239–244 (2005).
C. Shang, J. Rice, and J. Lin, Soil Sci. Soc. Am. J., 66, 1225–1230 (2002).
P. Praus, M. Turicova, S. Studentova, and M. Retz, J. Colloid Interface Sci., 304, 29–36 (2006).
T. Bezrodna, I. Chashechnikova, V. Nesprava, G. Puchkovska, Ye. Shaydyuk, Yu. Boyko J. Baran, and M. Drozd, Liq. Cryst., 37, 263–270 (2010).
I. Chashechnikova, L. Dolgov, T. Gavrilko, G. Puchkovska, Ye. Shaydyuk, N. Lebovka, V. Moraru, J. Baran, and H. Ratajczak, J. Mol. Struct., 744–747, 563–571 (2005).
A. V. Melezhik, Yu. I. Sementsov, and V. V. Yanchenko, Zh. Prikl. Khim., 78, No. 6, 938–944 (2005).
Yu. I. Tarasevich and F. D. Ovcharenko, Adsorption on Clay Minerals [in Russian], Naukova Dumka, Kiev (1975), pp. 3–5, 33–36, 53, 58–64, 75, 109–111, 239–243.
T. V. Bezrodna, G. V. Klishevich, V. I. Melnik, V. V. Nesprava, G. A. Puchkovska, and I. T. Chashechnikova, Zh. Prikl. Spektrosk., 77, No. 6, 770–774 (2010).
J. Madejova, Vib. Spectrosc., 31, 1–10 (2003).
N. D. Sokolov (ed.), Hydrogen Bond [in Russian], Nauka, Moscow (1981), pp. 112–155.
K. Suga and J. Rusling, Langmuir, 9, 3649–3655 (1993).
S. Makarenko and G. Puchkovska, Ukr. Fiz. Zh., 19, 421–426 (1974).
S. Makarenko and G. Puchkovska, Ukr. Fiz. Zh., 20, 476–483 (1975).
A. Babkov, G. Puchkovska, S. Makarenko, and T. Gavrilko, IR Spectroscopy of Molecular Crystals with Hydrogen Bonds [in Russian], Naukova Dumka, Kiev (1989), p. 30.
V. Vand, Acta Crystallogr., 4, 104–105 (1951).
G. Puchkovskaya, V. Danchuk, A. Kravchuk, and J. Kukielski, J. Mol. Struct., 704, 119–123 (2004).
T. Bezrodna, G. Puchkovska, V. Styopkin, J. Baran, M. Drozd, V. Danchuk, and A. Kravchuk, J. Mol. Struct., 973, 47–55 (2010).
I. Gnatyuk, N. Platonova, G. Puchkovska, E. Kotelnikova, S. Filatov, J. Baran, and M. Drozd, Zh. Strukt. Khim., 48, No. 4, 705–716 (2007).
H. Heinz, R. Vaia, R. Krishnamoorti, and B. Farmer, Chem. Mater., 19, 59–68 (2007).
L. Ricard, R. Cavagnat, and M. Rey-Lafon, J. Phys. Chem., 89, 4887–4894 (1985).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 78, No. 1, pp. 56–65, January–February, 2011.
Rights and permissions
About this article
Cite this article
Bezrodnaya, T.V., Nesprava, V.V., Puchkovskaya, G.A. et al. Structure and spectroscopic properties of organoclays doped by multiwall carbon nanotubes. J Appl Spectrosc 78, 50–58 (2011). https://doi.org/10.1007/s10812-011-9424-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-011-9424-y