Abstract
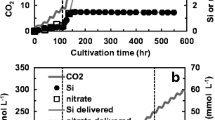
Diatoms are unicellular algae that make nanostructured biosilica shells called frustules possessing ordered pore arrays. Dissolved silicon (Si) in the form of Si (OH)4 is the required substrate for cell wall biosynthesis and cell division. The extracellular formation of β-chitin nanofibers by the centric diatom Cyclotella sp. was followed during batch cultivation of the cell suspension within a bubble-column photobioreactor at a low initial Si concentration (0.25 mM) leading to 1–2 cell divisions, and high Si concentration (1.7 mM) leading to 4–5 cell divisions. Fibers were excreted from 20 specialized pores (fultoportulae) lining the rim of each frustule valve face. In Si-limited batch cultivation, 80% of the fibers formed during the last cell division after dissolved Si was depleted from the medium. Within 24 h after the final cell division, fibers were nominally 56 nm diameter and 60 μm length, with 85% of the fibers between 20 and 80 μm, and one fiber per fultoportula was formed. After the final cell number density was maintained for 72 h, a second fiber per fultoportula was formed, presumably after breakage or scission of the first fiber from the cell. X-ray diffraction (XRD) analysis confirmed that isolated fibers were composed of pure β-chitin. The unprecedented length and purity of the β-chitin nanofibers suggest future advanced material applications.











Similar content being viewed by others
References
Aranaz I, Mengibar M, Harris R, Panos I, Miralles B, Acosta N, Galed G, Heras A (2009) Functional characterization of chitin and chitosan. Curr Chem Biol 3:203–230
Azuma K, Ifuku S, Osaki T, Okamoto Y, Minami S (2014) Preparation and biomedical applications of chitin and chitosan nanofibers. J Biomed Nanotechnol 10:2891–2920
Blackwell J, Parker KD, Rudall KM (1967) Chitin fibres of the diatoms Thalassiosira fluviatilis and Cyclotella cryptica. J Mol Biol 28:383–385
Chiriboga OG, Rorrer GL (2017) Control of chitin nanofiber production by the lipid-producing diatom Cyclotella sp. through fed-batch addition of dissolved silicon and nitrate in a bubble-column photobioreactor. Biotechnol Prog 33:407–415
Chiriboga O, Rorrer GL (2018) Effects of nitrogen delivery on chitin nanofiber production during batch cultivation of the diatom Cyclotella sp. in a bubble column photobioreactor. J Appl Phycol 30:1575–1581
Chiriboga O, Rorrer GL (2019) Phosphate addition strategies for enhancing the co-production of lipid and chitin nanofibers during fed-batch cultivation of the diatom Cyclotella sp. Algal Res 38:101403
Chisti Y, Moo-Young M (1989) On the calculation of shear rate and apparent viscosity in airlift and bubble column bioreactors. Biotechnol Bioeng 34:1391–1392
Ding F, Deng H, Du Y, Shi X, Wang Q (2014) Emerging chitin and chitosan nanofibrous materials for biomedical applications. Nanoscale 6:9477–9493
Dweltz NE, Colvin JR (1968) Studies on chitan (β-(1-4)-linked 2-acetamido-2-deoxy-D-glucan) fibers of the diatom Thalassiosira fluviatilis, Hustedt. III. The structure of chitan from x-ray diffraction and electron microscope observation. Can J Chem 46:1513–1521
Fischer TH, Valeri CR, Smith CJ, Scull CM, Merricks EP, Nichols TC, Demcheva M, Vournakis JN (2008) Non-classical processes in surface hemostasis: mechanisms for the poly-N-acetyl glucosamine-induced alteration of red blood cell morphology and surface prothrombogenicity. Biomed Mater 3:015009
Gügi B, Le Costaouec T, Burel C, Lerouge P, Helbert W, Bardor M (2015) Diatom-specific oligosaccharide and polysaccharide structures help to unravel biosynthetic capabilities in diatoms. Mar Drugs 13:5993–6018
Herth W (1979) The site of β-chitin fibril formation in centric diatoms. II. The chitin-forming cytoplasmic structures. J Ultrastruct Res 68:16–27
Herth W, Barthlott W (1979) The site of β-chitin fibril formation in centric diatoms. I. Pores and fibril formation. J Ultrastruct Res 68:6–15
Herth W, Zugenmaier P (1977) Ultrastructure of the chitin fibrils of the centric diatom Cyclotella cryptica. J Ultrastruct Res 61:230–239
Hildebrand M, Davis AK, Smith SR, Traller JC, Abbriano R (2012) The place of diatoms in the biofuels industry. Biofuels 3:221–240
Hockin NL, Mock T, Mulholland F, Kopriva S, Malin G (2012) The response of diatom central carbon metabolism to nitrogen starvation is different from that of green algae and higher plants. Plant Physiol 158:299–312
Huysman MJJ, Vyverman W, De Veylder L (2014) Molecular regulation of the diatom cell cycle. J Exp Bot 65:2573–2584
Imai T, Watanabe T, Yui T, Sugiyama J (2003) The directionality of chitin biosynthesis: a revisit. Biochem J 374:755–760
Jayakumar R, Prabaharan M, Nair SV, Tamura H (2010) Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv 28:142–150
Kaczmarska I, Beaton M, Benoit AC, Medlin LK (2005) Molecular phylogeny of selected members of the order Thalassiosirales (Bacillariophyta) and evolution of the fultoportula. J Phycol 42:121–138
Kato Y, Kitano K (1968) Solubility and dissolution rate of amorphous silica in distilled and sea water at 20 C. J Oceanogr Soc Jpn 24:147–152
Lebeau T, Robert JM (2003) Diatom cultivation and biotechnologically relevant products. Part II: current and putative products. Appl Microbiol Biotechnol 60:624–632
Levitan O, Dinamarca J, Hochman G, Falkowski PG (2014) Diatoms: a fossil fuel of the future. Trends Biotechnol 32:117–124
López-Rosales L, García-Camacho F, Sánchez-Mirón A, Contreras-Gómez A, Molina-Grima E (2017) Modeling shear-sensitive dinoflagellate microalgae growth in bubble column photobioreactors. Bioresour Technol 245:250–257
Martin-Jézéquel V, Hildebrand M, Brzezinski MA (2000) Silicon metabolism in diatoms: implications for growth. J Phycol 36:821–840
Muzzarelli RAA (2011) Chitin nanostructures in living organisms. In: Gupta NS (ed) Chitin, Topics in Geobiology. Springer Netherlands, Dordrecht, pp 1–34
Muzzarelli RAA, El Mehtedi M, Mattioli-Belmonte M (2014) Emerging biomedical applications of nano-chitins and nano-chitosans obtained via advanced eco-friendly technologies from marine resources. Mar Drugs 12:5468–5502
Nishiyama Y, Noishiki Y, Wada M (2011) X-ray structure of anhydrous β-chitin at 1 Å resolution. Macromolecules 44:950–957
Obata T, Fernie AR, Nunes-Nesi A (2013) The central carbon and energy metabolism of marine diatoms. Metabolites 3:325–346
Ogawa Y, Kimura S, Wada M (2011) Electron diffraction and high-resolution imaging on highly-crystalline β-chitin microfibril. J Struct Biol 176:83–90
Ogawa Y, Lee CM, Nishiyama Y, Kim SH (2016) Absence of sum frequency generation in support of orthorhombic symmetry of α-chitin. Macromolecules 49:7025–7031
Ozkan A, Rorrer GL (2017a) Effects of CO2 delivery on fatty acid and chitin nanofiber production during photobioreactor cultivation of the marine diatom Cyclotella sp. Algal Res 26:422–430
Ozkan A, Rorrer GL (2017b) Effects of light intensity on the selectivity of lipid and chitin nanofiber production during photobioreactor cultivation of the marine diatom Cyclotella sp. Algal Res 25:216–227
Pillai CKS, Paul W, Sharma CP (2009) Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci 34:641–678
Ragni R, Cicco SR, Vona D, Farinola GM (2018) Multiple routes to smart nanostructured materials from diatom microalgae: a chemical perspective. Adv Mater 30:1–23
Rolandi M, Rolandi R (2014) Self-assembled chitin nanofibers and applications. Adv Colloid Interf Sci 207:216–222
Rorrer GL (2018) Functionalization of frustules from diatom cell culture for optoelectronic properties. In: Losic D (ed) Diatom nanotechnology, progress and emerging applications, Nanoscienc. Royal Society of Chemistry Press, London, pp 79–110
Rorrer GL, Torres AJ, Durst R, Kelly C, Gale D, Maddux B, Ozkan A (2016) The potential of a diatom-based photosynthetic biorefinery for biofuels and valued co-products. Curr Biotechnol 5:237–248
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Smith SR, Abbriano RM, Hildebrand M (2012) Comparative analysis of diatom genomes reveals substantial differences in the organization of carbon partitioning pathways. Algal Res 1:2–16
Traller JC, Cokus SJ, Lopez DA, Gaidarenko O, Smith SR, McCrow JP, Gallaher SD, Podell S, Thompson M, Cook O, Morselli M, Jaroszewicz A, Allen EE, Allen AE, Merchant SS, Pellegrini M, Hildebrand M (2016) Genome and methylome of the oleaginous diatom Cyclotella cryptica reveal genetic flexibility toward a high lipid phenotype. Biotechnol Biofuels 9:1–20
Wang W, Gutu T, Gale DK, Jiao J, Rorrer GL, Chang C-H (2009) Self-assembly of nanostructured diatom microshells into patterned arrays assisted by polyelectrolyte multilayer deposition and inkjet printing. J Am Chem Soc 131:4178–4179
Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13:1133–1174
Funding
This work was supported by the US National Science Foundation (NSF), Emerging Frontiers for Research and Innovation program (EFRI), under award number 1240488.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
LeDuff, P., Rorrer, G.L. Formation of extracellular β-chitin nanofibers during batch cultivation of marine diatom Cyclotella sp. at silicon limitation. J Appl Phycol 31, 3479–3490 (2019). https://doi.org/10.1007/s10811-019-01879-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01879-6