Abstract
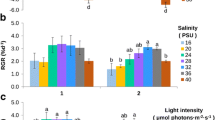
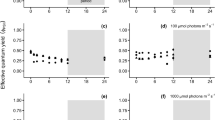
Gracilaria domingensis (Kützing) Sonder ex Dickie is widely distributed in the Brazilian coast and is one of a few Gracilaria species with occurrence in the southern region of the country. Its commercial importance is in the use in human food (in natura) and knowledge of the physiology is fundamental to make its cultivation successful. The objectives of the present work were to determine the limits of tolerance to temperature and irradiance variations and to evaluate comparatively their effects on growth rates and contents of total soluble proteins and pigments in female gametophytes of G. domingensis from Brazilian tropical region (ES strain) and from subtropical region (SC strain). Variations in temperature (from 15 to 35 °C) and irradiance (from 20 to 250 μmol photons m−2 s−1) were tested. Both strains tolerated temperature variation from 15 to 30 °C, but died at 35 °C. The ES strain had higher growth rates (from 3.1 to 6.1% day−1) in relation to the SC strain (from 1.3 to 4.1% day−1), but the two strains had the highest growth rate at 25 °C. The ES strain had higher concentrations of total soluble proteins at low temperature (15 °C), while the SC strain showed higher protein concentrations at high temperatures (25 and 30 °C). In addition, the chlorophyll a concentration of the ES strain was higher at 20 °C and lower at 30 °C. The irradiance variation influenced the growth rates of the two strains (from 2.7 to 6.0% day−1) and the lowest growth rates were observed at irradiance of 20 μmol photons m−2 s−1 and the highest growth rates were observed at a wide range of irradiance variation (from 40 to 250, and from 60 to 250 μmol photons m−2 s−1 for SC and ES strains, respectively). The studied strains of G. domingensis showed higher concentrations of total soluble protein at low irradiances (20, 40, and 80 μmol photons m−2 s−1). The highest concentrations of phycoerythrin (423.0 μg g−1 FW) and phycocyanin (215.3 μg g−1 FW) were observed in the ES strain grown at low irradiance (40 μmol photons m−2 s−1). The chlorophyll a concentration of the two strains decreased with increased irradiance, indicating a photoacclimation. Our results showed that the tropical strain of G. domingensis has higher growth rates over a broader temperature range than the subtropical strain, which could be an advantageous feature for maricultural purposes.






Similar content being viewed by others
References
Araújo FO, Ursi S, Plastino EM (2014) Intraspecific variation in Gracilaria caudata (Gracilariales, Rhodophyta): growth, pigment content and photosynthesis. J Appl Phycol 26:849–858
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buschmann A, Kuschel F, Vergara P, Schulz J (1992) Intertidal Gracilaria farming in southern Chile: differences of the algal provenience. Aquat Bot 42:327–337
Carnicas E, Jiménez C, Niell FX (1999) Effects of changes of irradiance on the pigment composition of Gracilaria tenuistipitata var. liui Zhang et Xia. J Photochem Photobiol B 50:149–158
Choi HG, Kim YS, Kim JH, Lee SJ, Park EJ, Ryu J, Nam KJ (2006) Effects of temperature and salinity on the growth of Gracilaria verrucosa and G chorda, with the potential for mariculture in Korea. J Appl Phycol 18:269–277
Faria AVF, Barufi JB, Plastino EM (2017) Ecotypes of Gracilaria caudata (Gracilariales, Rhodophyta): physiological and morphological approaches considering life history phases. J Appl Phycol 29:707–719
Guimarães SMPB (2003) Uma análise da diversidade da flora marinha bentônica do estado do Espírito Santo, Brasil. Hoehnea 30:11–19
Hayashi L, Bulboa C, Kradolfer P, Soriano G, Robledo D (2014) Cultivation of red seaweeds: a Latin American perspective. J Appl Phycol 26:719–727
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining clorophylls a, b, c 1, and c 2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Kakita H, Kamishima H (2006) Effects of environmental factors and metal ions on growth of the red alga Gracilaria chorda Holmes (Gracilariales, Rhodophyta). J Appl Phycol 18:469–474
Kursar TA, van Der Meer J, Alberte RS (1983) Light-harvesting system of red alga Gracilaria tikvahiae I. Biochemical analyses of pigment mutations. Plant Physiol 73:353–360
Lüning K (1990) Seaweeds: their environment biogeography and physiology. Wiley, New York 527p
Oliveira EC (1997) Macroalgas marinhas de valor comercial: técnicas de cultivo. Panorama da Aqüicultura 7:42–45
Oliveira EC, Plastino EM (1994) Gracilariaceae. In: Akatsuka I (ed) Biology economic algae. SPB Academic Publishing, The Hague, pp 185–226
Oliveira EC, Paula EJ, Plastino EM, Petti R (1995) Metodologias para o cultivo no axênico de macroalgas marinas in vitro. In: Alveal K, Ferrario ME, Oliveira EC, Sar E (eds) Manual de Métodos Ficológicos. Universidad de Concepción, Concepción, pp 429–455
Oliveira EC, Alveal K, Anderson R (2000) Mariculture of agar-producing Gracilarioid red algae. Rev Fish Sci 8:345–378
Orfanidis S, Breeman A (1999) Geographic variation in thermal traits in Digenea simplex and Champia parvula (Rhodophyta) in relation to present and glacial temperature regimes. J Phycol 35:919–930
Orfanidis S, Venekamp L, Breeman A (1999) Ecophysiological adaptations of two Mediterranean red algae in relation to distribution. Eur J Phycol 34:469–476
Pakker H, Breeman A (1996) Temperature responses of tropical to warm temperate seaweeds. II. Evidence for ecotypic differentiation in amphi-Atlantic tropical Mediterranean species. Eur J Phycol 31:133–141
Plastino EM, Guimarães M, Matioli SR, Oliveira EC (1999) Codominant inheritance of polymorphic color variants of Gracilaria domingensis Gracilariales, Rhodophyta. Genet Mol Biol 22:105–108
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29:2187–2200
Raikar SV, Iima M, Fujita Y (2001) Effect of temperature, salinity and light intensity on the growth of Gracilaria spp. (Gracilariales, Rhodophyta) from Japan, Malaysia and India. Indian J Mar Sci 30:98–104
Ramlov F, Souza JMC, Faria A, Maraschin M, Horta PH, Yokoya NS (2012) Effects of temperature, irradiance, and nutrients on the development of carposporelings and tetrasporophytes in Gracilaria domingensis (Kütz.) Sonder ex Dickie (Rhodophyta, Gracilariales). Bot Mar 55:253–259
Santelices B, Ugarte R (1990) Ecological differences among Chilean population of commercial Gracilaria. J Appl Phycol 2:17–26
Smit AJ (2004) Medicinal and pharmaceutical uses of seaweed natural products: a review. J Appl Phycol 16:245–262
Ursi S, Pedersén M, Plastino E, Snoeijs P (2003) Intraspecific variation of photosynthesis, respiration and photoprotective carotenoids in Gracilaria birdiae (Gracilariales: Rhodophyta). Mar Biol 142:997–1007
Wilson AJ, Critchley AT (1997) Studies on Gracilaria gracilis (Stackhouse) Steentoft, Farnham and Irvine and Gracilaria aculeata (Hering) Papenfuss from southern Africa. I. The influence of temperature, irradiance, salinity and nitrogen-nutrition on growth. S Afr J Bot 63:465–473
Yokoya NS (2000) Apical callus formation and plant regeneration controlled by plant growth regulators on axenic cultures of the red alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). Phycol Res 48:133–142
Yokoya NS, Oliveira EC (1992) Geographic distribution and growth responses to temperature variation of some South American red algae of economic importance. J Appl Phycol 4:339–345
Yokoya NS, Kakita H, Obika H, Kitamura T (1999) Effects of environmental factors and plant growth regulators on growth of the red alga Gracilaria vermiculophylla from Shikoku Island, Japan. Hydrobiologia 398/399:339–347
Yokoya NS, Necchi O, Martins AP, Gonzalez SF, Plastino EM (2007) Growth responses and photosynthetic characteristics of wild and phycoerythrin-deficient strains of Hypnea musciformis (Rhodophyta). J Appl Phycol 19:197–205
Yong YS, Yong WTL, Anton A (2013) Analysis of formulae for determination of seaweed growth rate. J Appl Phycol 25:1831–1834
Yoshimura CY, Cunha RS, Oliveira EC (2004) Testing open-water cultivation techniques for Gracilaria domingensis (Rhodophyta, Gracilariales) in Santa Catarina, Brazil. J Coast Res 39(SI):159–162
Zemke-White WL, Ohno M (1999) World seaweed utilization: an end-of-century summary. J Appl Phycol 11:369–376
Zou D, Gao K (2009) Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) grown at different irradiance levels. Phycologia 48:510–517
Acknowledgments
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for the scholarship of the first author and for research grant to NSY (CAPES/AUXPE-CIMAR 23038.001431/2014-75), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Proc. 310672/2016-3). The present study is part of the Master dissertation presented by the first author to the Graduate Program in Plant Biodiversity and Environment, Institute of Botany, São Paulo, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castro, J.Z., Yokoya, N.S. Growth and biochemical responses of tropical and subtropical strains of Gracilaria domingensis (Gracilariales, Rhodophyta) to temperature and irradiance variations. J Appl Phycol 31, 607–613 (2019). https://doi.org/10.1007/s10811-018-1520-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1520-4