Abstract
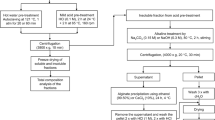
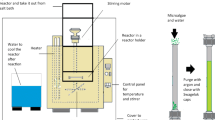
Hydrocarbons are easily extracted by organic solvents such as n-decane from wet samples of Botryococcus braunii by thermal pretreatment at 90 °C even after being cooled to room temperature. However, hydrocarbon recoveries are not as readily achieved at room temperature from samples pretreated at temperatures lower than 80 °C. This suggests that there is the point of no return for pretreatment temperature that enables effective solvent extraction of hydrocarbons at room temperature from wet algal samples of B. braunii. To elucidate the mechanism of hydrocarbon recovery from B. braunii following thermal pretreatments, we investigated the thermophysical properties of the water phase separated from heated algal slurry. Differential scanning calorimetry (DSC) measurements revealed sol–gel transitions in the water phase of algal slurry after protein denaturation at 64 °C in samples that was pretreated at 70 or 80 °C but not in those pretreated at 90 °C. Furthermore, the pretreated >70 °C water-soluble polymers revealed polysaccharides composed of galactose, arabinose, and uronic acid. These results suggest that the transition from sol state to gel state of water-soluble polysaccharides in algal slurry prevented hydrocarbon recovery with organic solvents since hydrocarbons were easily recovered from sol state samples pretreated at 70 or 80 °C when the extraction temperature was kept the same as the pretreatment temperature. These results reveal that the presence of water-soluble polymers with gelation ability in the water phase and removal of these polymers in sol state enable effective recovery of hydrocarbons at room temperature after thermal pretreatments.






Similar content being viewed by others
References
Aaronson S, Dubinsky Z (1982) Mass production of microalgae. Experientia 38:36–40
Atobe S, Saga K, Maeyama H, Fujiwara K, Okada S, Imou K (2014) Culture of the green microalga Botryococcus braunii Showa with LED irradiation eliminating violet light enhances hydrocarbon production and recovery. Biosci Biotechnol Biochem (in press)
Ben-Amotz A, Tornabene AG (1985) Chemical profile of selected species of microalgae with emphasis on lipids. J Phycol 21:72–81
Brown AC, Knights BA, Conway E (1969) Hydrocarbon content and its relationship to physiological state in the green alga Botryococcus braunii. Phytochemistry 8:543–547
Chanda SK, Hirst EL, Percival EGV, Ross AG (1939) The structure of alginic acid. Part II. J Am Chem Soc 1952:1833–1837
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Dodgson KS (1961) Determination of inorganic sulphate in studies on the enzymatic and nonenzymatic hydrolysis of carbohydrate and other sulphate esters. Biochem J 78:312–329
Dote Y, Sawayama S, Inoue S, Minowa T, Yokoyama S (1994) Recovery of liquid fuel from hydrocarbon-rich microalgae by thermochemical liquefaction. Fuel 73:1855–1857
Frenz J, Largeau C, Casadevall E, Kollerup F, Daugulis AJ (1989) Hydrocarbon recovery and biocompatibility of solvents for extraction from cultures of Botryococcus braunii. Biotechnol Bioeng 34:755–762
Furuhashi K, Saga K, Okada S, Imou K (2013) Seawater-cultured Botryococcus braunii for efficient hydrocarbon extraction. PLoS ONE 8:e66483
Hang A, Larsen B, Smidsrød O (1967) Studies on the sequence of uronic acid residues in alginic acid. Acta Chem Scand 21:691–704
Kita K, Okada S, Sekino H, Imou K, Yokoyama S, Amano T (2010) Thermal pre-treatment of wet microalgae harvest for efficient hydrocarbon recovery. Appl Energy 87:2420–2423
Largeau C, Casadevall E, Berkaloff C, Dhamelincourt P (1980) Sites of accumulation and composition of hydrocarbons in Botryococcus braunii. Phytochemistry 19:1043–1051
Magota A, Saga K, Okada S, Atobe S, Imou K (2012) Effect of thermal pretreatments on hydrocarbon recovery from Botryococcus braunii. Bioresour Technol 123:195–198
Metzger P, Berkaloff C, Casadevall E, Coute A (1985) Alkadiene- and botryococcene-producing races of wild strains of Botryoccus braunii. Phytochemistry 24:2305–2312
Miyoshi E, Takaya T, Nishinari K (1995) Gel-sol transition in gellan aqueous solutions. Macromol Symp 99:83–91
Nelson WL, Cretcher LH (1929) The alginic acid from Macrocystis pyrifera. J Am Chem Soc 51:1914–1922
Nelson WL, Cretcher LH (1932) The properties of d-mannuronic acid lactone. J Am Chem Soc 54:3409–3412
Nishinari K (2000) Hydrocolloids, Part 2, Fundamentals and applications in food, biology, and medicine. Elsevier, Amsterdam
Nonomura AM (1988) Botryococcus braunii var. showa (Chlorophyceae) from Berkeley, California, United States of America. Jpn J Phycol 36:285–291
Okada S, Murakami M, Yamaguchi K (1995) Hydrocarbon composition of newly isolated strains of the green microalga Botryococcus braunii. J Appl Phycol 7:555–559
Rinaudo M (1994) On the relation structure-properties of some polysaccharides used in the food industry. In: Nishinari K, Doi E (eds) Food Hydrocolloids- Structures, Properties, and Functions. Plenum Press, New York, pp 35–44
Saga K, Magota A, Atobe S, Okada S, Imou K, Osaka N, Matsui T (2013) Hydrocarbon recovery from concentrated algae slurry via thermal pretreatment. J Jpn Inst Energy 92:1214–1219
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Technical Report. NREL/TP-510–42618
Swale EMF (1968) The phytoplankton of Oak Mere, Cheshire, 1963-1966. Br Phycol Bull 3:441–449
Thomas WH, Seibert DLR, Alden M, Neori A, Eldridge P (1984) Yields, photosynthetic efficiencies and proximate composition of dense marine microalgal cultures. I. Introduction and Phaeodactylum tricormutum experiments. Biomass 5:181–209
Vaquez-Duhalt R, Arredondo-Vega BO (1991) Haloadaptation of the green alga Botryococcus braunii (race A). Phytochemistry 30:2919–2925
Wake LV, Hillen LW (1980) Study of a “bloomˮ of the oil-rich alga Botryococcus braunii in the Darwin River Reservoir. Biotechnol Bioeng 22:1637–1656
Wake LV, Hillen LW (1981) Nature and hydrocarbon content of blooms of the alga Botryococcus braunii occurring in Australian freshwater lakes. Aust J Mar Freshw Res 32:353–367
Yamaguchi K, Nakano II, Murakami M, Konosu S, Nakayama O, Kanda M, Nakamura A, Iwamoto H (1987) Lipid comparison of green alga, Botryococcus braunii. Agric Biol Chem 51:493–498
Acknowledgments
This study was performed as part of a project of the Strategic Development of Next-generation Bioenergy Utilization Technology, “Efficient Production of Hydrocarbon from Microalgae,” supported by the New Energy and Industrial Technology Development Organization (NEDO). We would like to thank all the people involved.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atobe, S., Saga, K., Hasegawa, F. et al. The effect of the water-soluble polymer released from Botryococcus braunii Showa strain on solvent extraction of hydrocarbon. J Appl Phycol 27, 755–761 (2015). https://doi.org/10.1007/s10811-014-0363-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0363-x