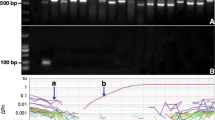
Abstract
A number of species belonging to the genus Alexandrium are among the main toxic microalgae responsible for Harmful Algal Blooms (HABs). The monitoring of coastal waters for the presence of these microalgae is essential to identify correlations between cell abundances and environmental factors that regulate bloom dynamics. In the attempt to improve the monitoring sensitivity and the rapidity at which a large number of field samples can be processed, several molecular methods for the detection of genetically distinct HAB species have been developed during the last years. In particular, real-time PCR has been shown to be a powerful method for quantitative detection of HAB species in environmental samples. When a plasmid is used as a standard, the knowledge of the amount of target gene per cell is essential for the determination of the cell number in the field sample. In this study, we analyzed the rRNA gene content variability in several Alexandrium catenella and Alexandrium taylori strains isolated from the Mediterranean Sea using a real-time PCR-based approach. The rRNA gene content was also analyzed in different growth phases, from early exponential to stationary conditions. The results showed a general variability in the rRNA gene content depending on the strain and, for the species A. taylori, in relation also to the growth phase. These results should be taken into account for the application of the real-time quantitative PCR-based techniques for monitoring purposes in coastal seawaters.




Similar content being viewed by others
References
Anderson DM (1998) Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions. In: Anderson DM, Cembella AD, Hallegraeff GM (eds) Physiological ecology of harmful algal blooms. Springer, Berlin, pp 29–48
Bertaux O, Mederic C, Valencia R (1991) Amplification of ribosomal DNA in the nucleolus of vitamin B12-deficient Euglena cells. Exp Cell Res 195:119–128 doi:10.1016/0014-4827(91)90507-Q
Caroppo C, Congestri R, Bruno M (2001) Dynamics of Dinophysis sensu lato species (Dinophyceae) in a coastal Mediterranean environment (Adiratic Sea). Cont Shelf Res 21:1839–1854 doi:10.1016/S0278-4343(01)00028-0
Coyne KJ, Handy SM, Demir E, Whereat EB, Hutchins DA, Portune KJ, Doblin MA, Cary SC (2005) Improved quantitative real-time PCR assays for enumeration of harmful algal species in field samples using an exogenous DNA reference standard. Limnol Oceanogr Methods 3:381–391
Dyhrman ST, Erdner D, La Du J, Galac M, Anderson DM (2006) Molecular quantification of toxic Alexandrium fundyense in the Gulf of Maine using real-time PCR. Harmful Algae 5:242–250 doi:10.1016/j.hal.2005.07.005
Emura A, Matsuyama Y, Oda T (2004) Evidence for the production of a novel proteinaceous hemolytic exotoxin by dinoflagellate Alexandrium taylori. Harmful Algae 3:29–37 doi:10.1016/j.hal.2003.08.004
Engberg J, Andersson P, Leick V, Collins J (1976) Free ribosomal DNA molecules from Tetrahymena pyriformis GL are giant palindromes. J Mol Biol 104:455–470 doi:10.1016/0022-2836(76)90281-3
Figueroa RI, Bravo I, Garcés E (2006) The multiple routes of sexuality in Alexandrium taylori (Dinophyceae) in culture. J Phycol 42:1028–1039 doi:10.1111/j.1529-8817.2006.00262.x
Findly RC, Gall JG (1978) Free ribosomal RNA genes in Paramecium are tandemly repeated. Proc Natl Acad Sci USA 75:3312–3316 doi:10.1073/pnas.75.7.3312
Fontaine M, Guillot E (2002) Development of a TaqMan quantitative PCR assay specific for Cryptosporidium parvum. FEMS Microbiol Lett 214:13–17 doi:10.1111/j.1574-6968.2002.tb11318.x
Galluzzi L, Penna A, Bertozzini E, Vila M, Garcés E, Magnani M (2004) Development of a real-time PCR assay for rapid detection and quantification of Alexandrium minutum (a dinoflagellate). Appl Environ Microbiol 70:1199–1206 doi:10.1128/AEM.70.2.1199-1206.2004
Garcés E, Masó M, Camp J (1999) A recurrent and localized dinoflagellate bloom in a Mediterranean beach. J Plankton Res 21:2373–2391 doi:10.1093/plankt/21.12.2373
Giacobbe MG, Yang XM (1999) The life history of Alexandrium taylori (Dinophyceae). J Phycol 35:331–338 doi:10.1046/j.1529-8817.1999.3520331.x
Giacobbe MG, Penna A, Gangemi E, Masó M, Garcés E, Fraga S, Bravo I, Azzaro F, Decembrini F, Penna N (2007) Recurrent high-biomass blooms of Alexandrium taylorii (Dinophyceae), a HAB species expanding in the Mediterranean Sea. Hydrobiologia 580:125–133 doi:10.1007/s10750-006-0459-7
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum, New York, pp 26–60
Hackett JD, Anderson DM, Erdner DL, Bhattacharya D (2004) Dinoflagellates: a remarkable evolutionary experiment. Am J Bot 91:1523–1534 doi:10.3732/ajb.91.10.1523
Hosoi-Tanabe S, Sako Y (2005) Species-specific detection and quantification of toxic marine dinoflagellates Alexandrium tamarense and A. catenella by Real-Time PCR assay. Mar Biotechnol 7:506–514 doi:10.1007/s10126-004-4128-4
Isbister GK, Kiernan MC (2005) Neurotoxic marine poisoning. Lancet Neurol 4:219–228 doi:10.1016/S1474-4422(05)70041-7
Kamikawa R, Hosoi-Tanabe S, Nagai S, Itakura S, Sako Y (2005) Development of a quantification assay for the cysts of the toxic dinoflagellate Alexandrium tamarense using real-time polymerase chain reaction. Fish Sci 71:987–991 doi:10.1111/j.1444-2906.2005.01055.x
Lim PT, Usup G, Leaw CP, Ogata T (2005) First report of Alexandrium taylori and Alexandrium peruvianum (Dinophyceae) in Malaysia waters. Harmful Algae 4:391–400 doi:10.1016/j.hal.2004.07.001
Moestrup O (2004) IOC Taxonomic Reference List of Toxic Plankton Algae, Intergovernmental Oceanographic Commission of UNESCO. http://ioc.unesco.org/hab/data.htm
Okamato OK, Hastings JW (2003) Genome-wide analysis of redox-regulated genes in a dinoflagellate. Gene 321:73–81 doi:10.1016/j.gene.2003.07.003
Penna A, Magnani M (1999) Identification of Alexandrium (Dinophyceae) species using PCR and rDNA-targeted probes. J Phycol 35:615–621 doi:10.1046/j.1529-8817.1999.3530615.x
Penna A, Galluzzi L (2008) PCR techniques as diagnostic tools for the identification and enumeration of toxic marine phytoplankton species. In: Evangelista V, Barsanti L, Frassanito AM, Passarelli V, Gualtieri P (eds) Algal toxins: nature, occurrence, effect and detection. NATO Book. Springer, Netherlands, pp 261–283
Penna A, Garcés E, Vila M, Giacobbe MG, Fraga S, Lugliè A, Bravo I, Bertozzini E, Vernesi C (2005) Alexandrium catenella (Dinophyceae), a toxic ribotype expanding in the NW Mediterranean Sea. Mar Biol (Berl) 148:13–23 doi:10.1007/s00227-005-0067-5
Penna A, Giacobbe MG, Penna N, Andreoni F, Magnani M (2002) Seasonal blooms of the HAB Dinoflagellate Alexandrium taylori Balech in a New Mediterranean Area (Vulcano, Aeolian Islands). PSZN Mar Ecol 23:1–9 doi:10.1111/j.1439-0485.2002.tb00030.x
Popels LC, Cary SC, Hutchins DA, Forbes R, Pustizzi F, Gobler CJ, Coyne KJ (2003) The use of quantitative polymerase chain reaction for the detection and enumeration of the harmful alga Aureococcus anophagefferens in environmental samples along the United States East Coast. Limnol Oceanogr Methods 1:92–102
Prokopowich CD, Gregory TR, Crease TJ (2003) The correlation between rDNA copy number and genome size in eukaryotes. Genome 46:48–50 doi:10.1139/g02-103
Ravel-Chapuis P, Nicolas P, Nigon V, Neyret O, Freyssinet G (1985) Extrachromosomal circular nuclear rDNA in Euglena gracilis. Nucleic Acids Res 13:7529–7537 doi:10.1093/nar/13.20.7529
Saito K, Drgon T, Robledo JA, Krupatkina DN, Vasta GR (2002) Characterization of the rRNA locus of Pfiesteria piscicida and development of standard and quantitative PCR-based detection assays targeted to the nontranscribed spacer. Appl Environ Microbiol 68:5394–5407
Scholin CA, Anderson DM, Sogin ML (1993) Two distinct small-subunit ribosomal RNA genes in the North American toxic dinoflagellate Alexandrium fundyense (Dinophyceae). J Phycol 29:209–216 doi:10.1111/j.0022-3646.1993.00209.x
Shumway SE (1990) A review of the effects of algal blooms on shellfish and aquaculture. J World Aquacult Soc 21:65–104 doi:10.1111/j.1749-7345.1990.tb00529.x
Spector DL (1984) Dinoflagellate nuclei. In: Spector DL (ed) Dinoflagellates. Academic, Orlando, Florida, USA, pp 107–147
Throndsen J (1978) Preservation and storage. In: Sournia A (ed) Phytoplankton manual. UNESCO, Paris, pp 69–74
Vila M, Camp J, Garcés E, Masó M, Delgado M (2001) High resolution spatio-temporal detection of HABs in confined waters of the NW Mediterranean. J Plankton Res 23:497–514 doi:10.1093/plankt/23.5.497
Wong JTY, New DC, Wong JCW, Hung VKL (2003) Histone-like proteins of the dinoflagellate Crypthecodinium cohnii have homologies to bacterial DNA-binding proteins. Eukaryot Cell 2:646–650 doi:10.1128/EC.2.3.646-650.2003
Yeung PK, Kong KF, Wong FT, Wong JT (1996) Sequence data for two large-subunit rRNA genes from an Asian strain of Alexandrium catenella. Appl Environ Microbiol 62:4199–4201
Zhu F, Massana R, Not F, Marie D, Vaulot D (2005) Mapping of picoeucaryotes in marine ecosystems with quantitative PCR of the 18S rRNA gene. FEMS Microbiol Ecol 52:79–92 doi:10.1016/j.femsec.2004.10.006
Acknowledgements
We would like to thank M. G. Giacobbe, S. Fraga and D.M. Anderson for supplying culture strains and M. Vila for the contribution in field sampling. This work was partially supported by the EU STRATEGY project contract n. EVK3-CT-2001-00046, EU SEED project contract n. GOCE-CT-2005-003875 and EU NACBO project 500804-2 (2004).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1
The rRNA gene content in nine A. catenella strains was determined in exponentially growing cells. A statistically significant rRNA gene content variability (ANOVA, p < 0.01) has been generally found. Moreover, the strains containing similar (not significantly different, p > 0.01) amount of rRNA gene copies cell−1 have been identified and grouped as follows: strains CNRACATC2, VGO605, CSIC-C7 (first group); strain CNR-ACATA4 (second group); strains CNRACATS2, AC2C, VGO563, CNR-ACATS1 (third group); strain CNR-ACATS3 (fourth group). The A. catenella strains were isolated from: Tyrrhenian Sea, Olbia, Italy (white columns); Catalan Sea, Barcelona, Spain (grey columns); Catalan Sea, Tarragona, Spain (dark columns). The results of the statistical analysis showed that the rRNA gene content was independent to the geographical origin of the different strains (PDF 27.2 KB)
Rights and permissions
About this article
Cite this article
Galluzzi, L., Bertozzini, E., Penna, A. et al. Analysis of rRNA gene content in the Mediterranean dinoflagellate Alexandrium catenella and Alexandrium taylori: implications for the quantitative real-time PCR-based monitoring methods. J Appl Phycol 22, 1–9 (2010). https://doi.org/10.1007/s10811-009-9411-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-009-9411-3