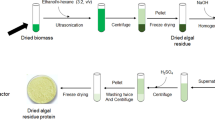
Abstract
Comparison of data of protein content in algae is very difficult, primarily due to differences in the analytical methods employed. The different extraction procedures (exposure to water, grinding, etc.), protein precipitation using different amounts of 25% trichloroacetic acid and quantification of protein by two different methods and using two protein standards were evaluated. All procedures were tested using freeze-dried samples of three macroalgae: Porphyra acanthophora var. acanthophora, Sargassum vulgare and Ulva fasciata. Based on these results, a protocol for protein extraction was developed, involving the immersion of samples in 4.0 mL ultra-pure water for 12 h, followed by complete grinding of the samples with a Potter homogeniser. The precipitation of protein should be done with 2.5:1 25% TCA:homogenate (v/v). The protocol for extraction and precipitation of protein developed in this study was tested with other macroalgae (Aglaothamnion uruguayense, Caulerpa fastigiata, Chnoospora minima, Codium decorticatum, Dictyota menstrualis, Padina gymnospora and Pterocladiella capillacea) and microalgae (Amphidinium carterae, Dunaliella tertiolecta, Hillea sp., Isochrysis galbana and Skeletonema costatum). Comparison with the actual protein content determined from the sum of amino acid residues, suggests that Lowry's method should be used instead of Bradford's using bovine serum albumin (BSA) as protein standard instead of casein. This may be related to the reactivity of the protein standards and the greater similarity in the amino acid composition of BSA and algae. The current results should contribute to more accurate protein determinations in marine algae.
Similar content being viewed by others
References
Berges JA, Fischer AE, Harrison PJ (1993) A comparison of Lowry, Bradford and Smith protein assays using different protein standards and protein isolated from marine diatom Thalassiosira pseudonana. Mar. Biol. 115: 187– 193.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Brown MR (1991) The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 145: 79–99.
Clayton JR Jr, Dortch Q, Thoresen SS, Ahmed SI (1988) Evaluation of methods for the separation and analysis of proteins and free amino acids in phytoplankton samples. J. Plankton Res. 10: 341–358.
Compton SJ, Jones CG (1985) Mechanism of dye response and interference in the Bradford protein assay. Anal. Biochem. 151: 369–374.
Conklin-Brittain NL, Dierenfeld ES, Wrangham RW, Norconk M, Silver SC (1999) Chemical protein analysis: A comparison of Kjeldahl crude protein and total ninhydrin protein from wild, tropical vegetation. J. Chem. Ecol. 25: 2601–2622.
Crossman DJ, Clements KD, Cooper GJS (2000) Determination of protein for studies of marine herbivory: A comparison of methods. J. Exp. Mar. Biol. Ecol. 244: 45–65.
Dortch Q, Clayton JR Jr, Thoresen SS, Ahmed SI (1984) Species differences in accumulation of nitrogen pools in phytoplankton. Mar. Biol. 81: 237–250.
Eze JMO, Dumbroff EB (1982) A comparison of the Bradford and Lowry methods for the analysis of protein in chlorophyllous tissue. Can. J. Bot. 60: 1046–1049.
Ezeagu IE, Petzke JK, Metges CC, Akinsoyinu AO, Ologhobo AD (2002) Seed protein contents and nitrogen-to-protein conversion factors for some uncultivated tropical plant seeds. Food Chem. 78: 105–109.
Fleurence J (1999) The enzymatic degradation of algal cell walls: A useful approach for improving protein accessibility? J. Appl. Phycol. 11: 313–314.
Fleurence J, Le Couer C, Mabeau S, Maurice M, Landrein A (1995) Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol. 7: 577–582.
Folin O, Ciocalteu V (1927) On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 73: 627–650.
Fujihara S, Kasuga A, Aoyagi Y (2001) Nitrogen-to-protein conversion factors for common vegetables in Japan. J. Food Sci. 66: 412–415.
Galland-Irmouli A-V, Fleurence J, Lamghari R, Luçon M, Rouxel C, Barbaroux O, Bronowicki J-P, Villaume C, Guéant J-L (1999) Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J. Nutr. Biochem. 10: 353–359.
Heidelbaugh ND, Huber CS, Bednarczyk JF, Smith MC, Rambaut PC, Wheeler HO (1975) Comparison of three methods for calculating protein content of foods. J. Agric. Food Chem. 23: 611–613.
Hein M, Pedersen MF, Sand-Jensen K (1995) Size-dependent nitrogen uptake in micro- and macroalgae. Mar. Ecol. Prog. Ser. 118: 247–253.
Kaehler S, Kennish R (1996) Summer and winter comparisons in the nutritional value of marine macroalgae. Hong Kong Bot. Mar. 39: 11–17.
Kester DR, Duedale IW, Connors DN, Pytkowicz RM (1967) Preparation of artificial sea water. Limnol Oceanogr. 12: 176–179.
Lee RE (1999) Phycology. 3rd ed., Cambridge University Press, Cambridge, 614 pp.
Legler G, Müller-Platz CM, Mentges-Hettkamp M, Pflieger G, Jülich E (1985) On the chemical basis of the Lowry protein determination. Anal. Biochem. 150: 278–287.
Lourenço SO, Barbarino E, De-Paula JC, Pereira LOS, Lanfer Marquez UM (2002) Amino acid composition, protein content, and calculation of nitrogen-to-protein conversion factors for nineteen tropical seaweeds. Phycol. Res. 50: 233–241.
Lourenço SO, Barbarino E, Lanfer Marquez UM, Aidar E (1998) Distribution of intracellular nitrogen in marine microalgae: Basis for the calculation of specific nitrogen-to-protein conversion factors. J. Phycol. 34: 798–811.
Lourenço SO, Barbarino E, Lavín PL, Lanfer Marquez UM, Aidar E (2004) Distribution of intracellular nitrogen in marine microalgae. Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 39: 17–32.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J. Biol. Chem. 193: 265–275.
Mattoo RL, Ishaq M, Saleemuddin M (1987) Protein assay by Coomassie Brilliant Blue G-250-binding method is unsuitable for plant tissues rich in phenols and phenolases. Anal. Biochem. 163: 376–384.
McDermid KJ, Stuercke B (2003) Nutritional composition of edible Hawaiian seaweeds. J. Appl. Phycol. 15: 513–524.
McHugh DJ (1991) Worldwide distribution of commercial resources of seaweeds including Gelidium. Hydrobiologia 221: 19–29.
Morrison RT, Boyd RN (2003) Organic Chemistry, 7th ed., Allyn and Bacon, Boston, 1200 pp.
Mossé J (1990) Nitrogen to protein conversion factor for ten cereals and six legumes or oilseeds. A reappraisal of its definition and determination. Variation according to species and to seed proteic content. J. Agric. Food Chem. 38: 18–24.
Nguyen RT, Harvey HR (1994) A rapid micro-scale method for the extraction and analysis of protein in marine samples. Mar. Chem. 45: 1–14.
Oliveira EC, Corbisier TN, de Eston VR, Ambrosio O (1997) Phenology of a seagrass (Halodule wrightii) bed on the southeast coast of Brazil. Aqua. Bot. 56: 25–33.
Ovalle ARC, Rezende CE, Carvalho CEV, Jennerjahn TC, Ittekkot V (1999) Biogeochemical characteristics of coastal waters adjacent to small river-mangrove systems, East Brazil Geo-Mar. Lett. 19: 179–185.
Pérez-Lloréns JL, Benítez E, Vergara JJ, Berges JA (2003) Characterization of proteolytic enzyme activities in macroalgae. Eur. J. Phycol. 38: 55–64
Peterson GL (1979) Review of the folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal. Biochem. 100: 201–220.
Ramos MV, Monteiro ACO, Moreira RA, Carvalho AFFU (2000) Amino acid composition of some Brazilian seaweed species. J. Food Biochem. 24: 33–39.
Stoscheck CM (1990) Quantitation of protein. Methods Enzymol. 182: 50–68.
Walne PR (1966) Experiments in the large scale culture of the larvae of Ostrea edulis. Fishery Invest. (Lond.) (Ser 2) 25(4): 1–53.
Wong KH, Cheung CK (2000) Nutritional evaluation of some subtropical red and green seaweeds Part I—proximate composition, amino acid profiles and some physico-chemical properties. Food Chem. 71: 475–482.
Wong KH, Cheung CK (2001) Nutritional evaluation of some subtropical red and green seaweeds Part II. In vitro protein digestibility and amino acid profiles of protein concentrates. Food Chem. 72: 11–17.
Wynne MJ (1998) A checklist of benthic marine algae of the tropical and subtropical western Atlantic: First revision. Nova Hedwigia 116: 1–155.
Zamer WE, Shick JM, Tapley DW (1989) Protein measurement and energetic considerations: Comparisons of biochemical and stoichiometric methods using bovine serum albumin and protein isolated from sea anemones. Limnol. Oceanogr. 34: 256– 263.
Zar JH (1996) Biostatistical Analysis. 3rd ed., Prentice Hall, Inc., Upper Saddle River, USA. 920 pp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbarino, E., Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J Appl Phycol 17, 447–460 (2005). https://doi.org/10.1007/s10811-005-1641-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10811-005-1641-4