Abstract
Participants with autism spectrum disorder (ASD) (n = 121, mean [SD] age: 14.6 [8.0] years) and typically developing (TD) controls (n = 40, 16.4 [13.3] years) were presented with a series of videos representing biological motion on one side of a computer monitor screen and non-biological motion on the other, while their eye movements were recorded. As predicted, participants with ASD spent less overall time looking at presented stimuli than TD participants (P < 10–3) and showed less preference for biological motion (P < 10–5). Participants with ASD also had greater average latencies than TD participants of the first fixation on both biological (P < 0.01) and non-biological motion (P < 0.02). Findings suggest that individuals with ASD differ from TD individuals on multiple properties of eye movements and biological motion preference.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Infants typically show an early visual preference for the movement of other human beings (Blake and Shiffrar 2007; Simion et al. 2008). This attention and orientation to biological motion may be heritable, and could be a precursor to development of socio-cognitive abilities (Wang et al. 2018). Preference for biological motion may not be present to the same extent in autism spectrum disorder (ASD) (Klin et al. 2009; Pierce et al. 2011). Lack of attention to social information, in particular biological motion, may be one of the mechanisms leading to the social deficits that are characteristic of ASD (Chevallier et al. 2012; Franchini et al. 2016).
Eye-tracking (ET) measures are commonly used to determine focus of attention in passive viewing tasks. Reviews of studies of social attention in ASD have found that attention to social information is the largest discriminator between ASD and typically developing (TD) groups (2016; Frazier et al. 2017), and therefore has potential as a diagnostic biomarker, and may possibly be used as an early and independent measure of symptom severity or change in clinical trials (Dawson et al. 2012; Murias et al. 2018). At a low level, biological motion can be represented by simple point-light displays (PLD), which show movement of major joints and no other visible features (Johansson 1973; Johnson 2006). The majority of studies testing biological motion orientation and deficits in ASD make use of PLD.
Preferential viewing tasks show PLD figures that are in engaged in repetitive human activity, presented alongside scrambled dots, inverted figures, or rotating dots. Several studies found that young children with ASD, unlike TD individuals, do not preferentially attend to PLD biological motion (Annaz et al. 2012; Klin et al. 2009; Wang et al. 2015). Other studies fail to replicate between-group differences, and in some cases report a stronger preference for biological motion in preschool children with ASD (Fujisawa et al. 2014). Very few studies report on attention to PLD biological motion in older individuals with ASD. Between-group differences in viewing time of biological motion were not observed in these limited reports of adolescents and adults with ASD, leading to the supposition that preference might become less prominent with age (Fujioka et al. 2016). Other studies have found that reduced visual sensitivity to biological motion is maintained into adulthood indicating a continuation of deficits, not necessarily detected by visual preference tasks for socially relevant information (Kaiser et al. 2010). There are also indications that intelligence quotient (IQ) can impact perception of biological motion and may moderate responses in ASD (Rutherford and Troje 2012).
Beyond considering attention to biological motion as a discriminator between ASD and TD groups, biological motion has potential as an indicator, or experimental biomarker, for change in response to intervention in ASD. There is some evidence that attention to PLD biological motion may be sensitive to changes in social cognition; for example, TD adults administered oxytocin showed increased perception of PLD biological motion (Kéri and Benedek 2009). These attention changes detected by ET may be linked to neurophysiology and modulation of an electroencephalogram, wherein enhanced suppression in mu and beta bands are associated with biological motion and social stimuli perception (Perry et al. 2010). Interestingly, neural responses to biological motion are found to vary with autistic-like traits in TD adults (Puglia and Morris 2017). Moreover, in an ASD group of 19 high-functioning males without intellectual disability, a significant increase was found in first orientation to biological motion, but not latency or time spent looking, in a group administered a vasopressin 1a receptor antagonist compared to placebo (Umbricht et al. 2017).
In addition to differences in preference for biological motion, it is possible that non-social components of attention unrelated to social motivation may contribute to differences observed in passive viewing tasks in ASD. For example, overall attention to stimuli, irrespective of content, has been found to be lower in individuals with ASD compared to TD individuals (Campbell et al. 2014; Chawarska et al. 2012, 2016, 2014; Shic et al. 2011). Further, individuals with ASD compared to TD individuals may process images in different ways, and thus characteristics such as number of shifts and latency of shifts in attention may differ (Keehn et al. 2013; Landry and Bryson 2004; Sacrey et al. 2014; Sasson et al. 2008, 2011). Differences in viewing patterns, e.g., longer fixation times, relate to symptoms such as repetitive behaviors in ASD (Manyakov et al. 2018).
ET studies of social and non-social attention in ASD vary in terms of age of participants, with most studies focusing on younger children and infants and much fewer on adults (Guillon et al. 2014). Findings are rarely replicated, and magnitude of effects differs widely (Frazier et al. 2017). Research in ASD is currently focused on identification of biomarkers that may be objective, cost effective and viable diagnostic or change measures. These biomarkers may serve as a proxy for social functioning and could prove useful as endpoints in clinical trials. In order to identify such biomarkers, it is important to have both a replication of previous findings (Bradshaw et al. 2019; Klin 2018; McPartland 2017; Murias et al. 2018) and an understanding of the developmental trajectory of differences in allocation of social attention between ASD and TD groups.
Methods
Participants
Participants aged ≥ 6 years with a confirmed diagnosis of ASD based on clinical examination, caregiver interview and use of the Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2) (Lord et al. 2012) were enrolled. Key exclusion criteria were a measured composite score on the Kaufmann Brief Intelligence Test-2 (KBIT-2) (Kaufman and Kaufman 2004) of < 60, and history of or current significant medical illness. Each site also enrolled a control sample of TD participants, aged ≥ 6 years, with a score in the normal range on the Social Communication Questionnaire (Rutter et al. 2003) who did not meet criteria for any major mental health disorder (American Psychiatric Association 2013) assessed using the Mini-International Neuropsychiatric Interview (MINI) (Hergueta et al. 1998) for those 18 years old and above, and MINI-KID caregiver interview (Sheehan et al. 2010) for participants under 18 years. There were no exclusion criteria based specifically on vision. If participants required corrective lenses to view the screen and were able to obtain calibration on the measure at the start of each test set, then their eye-tracking data were included in the study.
In total, 136 individuals with ASD and 41 TD controls completed the study. After exclusions due to technical or calibration failures, the study population included 121 (89.0%) individuals with ASD and 40 (97.6%) TD controls. Table 1 lists characteristics of each analyzed group of participants. Further details on the participant characteristics can be found in Ness et al. (2019). Note that no socioeconomic data on the participants were collected.
Biological Motion
The biological motion task consisted of videos in which two PLD animations were shown side-by-side. A PLD representing biological motion was shown on one side and a non-biological PLD was shown on the other. These stimuli were previously used by and reported by Umbricht et al. (2017). The PLDs were made up of dynamic black-point displays on a grey background (Supplementary Fig. 1). Each video lasted approximately four seconds. The biological/non-biological side was counterbalanced across presentations. Animations of biological motion were derived from a human actor’s performance and retrieved from the Carnegie Mellon University (CMU) Motion Capture Database (CMU Graphics Lab 2011). In contrast, animations of non-biological motion were computer-generated animations of moving dots, either phase-scrambled versions of the biological motion or continuous rotation (i.e. spinning around the vertical axis) of the first frame of the biological motion animation (see Supplementary Material for the analysis of attention to the two types of non-biological motion stimuli). All stimuli contained the same number of dots.
Biological and non-biological motion stimuli were matched in terms of motion complexity and speed of movement (see also Supplementary Material). There was an equal amount of novel stimuli in both the biological and non-biological motion condition. Both phase-scrambled and rotational control conditions were created using an original biological motion stimulus as a template. Phase-scrambled motion was created by time shifting each biological-motion point’s motion by a random time offset. These time offsets were randomly selected to sample uniformly between − 417 and 417 ms based on the number of points shown. The periodicity of this offset (833 ms) was ascertained by examining the dominant period of biological motion movement across clips (approximately 100 frames at 120 frames per second) via autocorrelation. This process guaranteed the total relative local motion of individual point-lights was comparable between phase-scrambled and unscrambled biological motion stimuli. The rotational point-light display was created by computing the dominant periodicity of hip movement in the source biological motion stimuli via autocorrelation and then rotating the first frame of the biological motion stimulus about the z-axis (following the spine in upright walking) at a constant speed given by hip periodicity. Stimuli were designed so that the first frame shown would be comparable across all conditions (unscrambled biological motion, scrambled biological motion, rotation).
This biological motion task was part of a large, observational, multi-center study conducted from 06 July 2015 to 14 October 2016 at nine study sites in the US (ClinicalTrials.gov, NCT02668991) and consisted of passive viewing tasks (Bangerter et al. 2020a, 2020b; Jagannatha et al. 2019; Manfredonia et al. 2018; Manyakov et al. 2018; Ness et al. 2019; Sargsyan et al. 2019). In this study, both groups of participants completed the same set of biosensor tasks. The total viewing time was approximately 40 min, including videos and other static stimuli presentations, divided into three sets, between which participants were allowed to take a break. Sixty different videos of biological motion were interspersed between these other stimuli and presented in 6 blocks of 10 videos (2 blocks within each set). An inter-stimulus slide that contained a cartoon image in the center of a grey screen was used for a duration of 1.5–2 s to re-orientate participants towards the center of the screen before each video, though no control was performed to verify the latter.
Behavior Rating Scales
Scale data were collected concurrently with eye-tracking data. Parents or caregivers of individuals with ASD were required to spend at least 3 days per week with participants. They completed the following scales:
-
Autism Behavior Inventory (ABI), a rating scale developed to assess change in core and associated symptoms of autism (Bangerter et al. 2017).
-
Aberrant Behavior Checklist—Community (ABC) assesses general behaviors (Aman et al. 2004; Aman and Singh 2017).
-
Child Adolescent Symptom Inventory-Anxiety (CASI-Anx) is a 20-item subset of the CASI which assesses anxiety (Gadow and Sprafkin 1997).
-
Social Responsiveness Scale 2™ (SRS-2) identifies presence and severity of social impairment due to ASD (Constantino et al. 2003).
-
Repetitive Behavior Scale—Revised (RBS-R) provides a quantitative measure of the full spectrum of repetitive behaviors (Bodfish et al. 1999).
Each of the above scales and ADOS-2 consist of several subscales that reflect different ASD symptoms. All subscales used in this study are presented in Table 2. Standard scores for the above scales and the calibrated severity score of the ADOS-2 were used in the reported analyses.
Procedure
Participants sat in a comfortable chair approximately 60 cm from a 23-inch computer screen (1920 × 1080 pixels). The height of the chair and screen were adjusted to ensure that participants’ eyes were level with the center of the screen. ET data were collected using the Tobii X2 eye tracker, with a sampling rate of 30 Hz, mounted below the screen. iMotions Biometric Research Platform (https://imotions.com/) was used for stimuli presentation, data synchronization, and automatic calibration. Participants were allowed to freely observe presented stimuli. To ensure a high accuracy of the eye movement recordings (e.g., Blignaut and Wium 2014), before each experimental set a five-point calibration procedure consisting of animated cartoon characters paired with an auditory cue was performed. The calibration procedure was aimed to reach the mean distance between the participant’s gaze direction and the target points of less than 0.5° of visual angle.
Data Analysis
For each frame, circles with the radius of approximately 3.7° of visual angle centered at each “black dot” corresponding to (non-)biological motion were drawn. Combination of such circles that corresponded to dots representing biological motion determined a biological motion region of interest (ROI). Similarly, a non-biological motion ROI was determined. Supplementary Fig. 1 shows two dynamic ROIs on a single example frame. Fixations were identified using the Binocular-Individual Threshold algorithm (van der Lans et al. 2011). The minimum required number of tracked samples and the maximum acceptable number of consecutive untracked samples in the eye tracking signal in a single fixation were both set to three. The following eye movement metrics were computed:
-
Total valid time (%)—percentage of time participant gazed at the screen, which is the opposite to data loss.
-
Preference for biological motion (%)—ratio of total time a participant spent looking at biological motion to total time he/she spent looking at biological and non-biological motion together.
-
Percentage of time the first fixation was on biological motion (%)—percentage of time participant first fixated on biological rather than on non-biological motion.
-
Average latency of the first fixation on biological (non-biological) motion (msec)—averaged (across all videos) time from stimulus onset until participant fixated for the first time on a ROI defining biological (non-biological) motion. Note that no control was performed to verify participant’s attention on the center of the screen in the beginning of each video stimulus presentation.
Analysis of covariance (ANCOVA) was conducted to compare eye movement metrics between the ASD and TD groups. Specifically, a single eye movement metric was used as a dependent variable, whereas participant group served as an independent variable. Participant’s age and gender entered the analysis as additional factors. Note that the KBIT-2 test was only administered in the ASD group and, thus, participant’s IQ score could not be included in the analysis as an additional factor. Supplementary Tables 6 and 7 show the results of tests for the homogeneity of variance assumption made in the ANCOVA models, and the results obtained with the same ANCOVA models as correcting for the violations of the assumption, respectively. Since ANCOVA models including two-factor interactions between participant group and the two other covariates did not significantly differ from those without any interaction (analysis of variance: all P-values > 0.18), statistical inference was based on the models without interactions. Note also that none of the scale data (see Behavior Rating Scales) entered any of the ANCOVA models. To test for effect of outliers on the obtained results, the same analyses were repeated after removing potential outliers from the analyzed dataset (Supplementary Table 1). To test for effect of site on the eye movement metrics, for each metric we conducted ANCOVA similar to that described above but additionally included a site identifier as an independent categorical variable. Similarly, to test for an interaction between participant’s age and group, the original ANCOVA models included an interaction term between these two factors. To test for effect of IQ on the differences in eye movement metrics between the two groups of participants, the entire ASD sample was divided into three separate groups based on the level of IQ. The tested levels represented the ASD participants with a low (range of the KBIT-2 IQ composite score: 60–84; n = 34 participants), average/normal (85–115; n = 63), and high (116–136; n = 24) IQ. The same ANCOVA’s as above were conducted combining the data of all TD participants and individuals with ASD with a specific level of IQ (Supplementary Table 2). The effect size of participant group on each eye movement metric was estimated using Cohen’s d and partial eta-squared (ηp2).
The Wilcoxon signed rank test was applied to compare preference for biological motion and “% time the first fixation was on biological motion” against the chance level (50%) in each of the two groups of participants separately. Similarly, to compare average latency of the first fixation between biological and non-biological stimuli within a single group of participants, the Wilcoxon matched pairs signed rank test was used.
Relationships between the eye movement metrics and different ASD symptoms were assessed using Spearman partial correlations, with participant’s age, gender and KBIT-2 IQ composite score serving as covariates (Supplementary Table 5). Spearman correlations were also used to assess relationships between the eye movement metrics and KBIT-2 IQ composite score (Supplementary Table 3) as well as between preference for biological motion and participant’s age (Supplementary Fig. 2). All reported correlations (rS) were computed using the data of all ASD participants. The choice of Spearman correlations was attributed to a generally lower susceptibility of this type of correlations to potential outliers present in the data, as compared to Pearson correlations. Note, however, that qualitatively similar results were also observed when using Pearson correlations.
All statistical analyses were performed in MATLAB and R, with package “ppcor” in R used to compute partial correlations (Kim 2015). All reported P-values were two-sided, except those generated by ANCOVAs and based on F-statistic. The P-values corresponding to the differences in eye movement metrics between the two groups of participants were adjusted for multiple comparisons (five tests) using the Benjamini–Hochberg procedure (false discovery rate = 5%) and are reported as such throughout the main text. Non-adjusted, original P-values of all ANCOVA’s are listed in Supplementary Tables 1 and 2. No other correction for multiple comparisons was performed, since the study was exploratory rather than hypothesis-driven. Note that the number of statistical comparisons and, thus, the exact cut-off for significant P-values in each analysis was debatable. For example, the total number of tests entering the only reported adjustment for multiple comparisons (see above) was set to five. Alternatively, it could have been set to 15 when additionally counting the tests run to assess the effect of participant’s gender and age on the observed results. Adjustment for multiple comparisons for the relationships between the eye movement metrics and ASD symptoms (Supplementary Table 5) could have been done in multiple ways: for each symptom and behavior rating scale separately but across all five eye movement metrics, for each eye movement metric separately but across all symptoms and behavior rating scales, and combining all tests regardless eye movement metric, symptom and behavior rating scale. For the reasons outlined above, the P-values were reported “as is” with values < 0.05 considered significant.
Results
Comparison of Eye Movement Metrics Between ASD and TD Participants
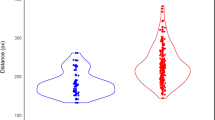
Differences in eye movement metrics between ASD (n = 121 participants) and TD (n = 40) groups are shown in Fig. 1. Analysis of covariance revealed a significant effect of participant group on all metrics (all P-values corrected for multiple comparisons < 0.03), except “% time the first fixation was on biological motion” (Supplementary Table 1). On average, the ASD group showed a significantly lower level of visual attention to presented stimuli than the TD group (% total valid time; ASD vs. TD, mean: 80.0% vs. 89.2%; Cohen’s d = 0.68, ηp2 = 0.08, P < 10–3). In comparison to the TD group, the ASD group spent significantly less time looking at stimuli that depicted biological as opposed to non-biological motion (% preference for biological motion; ASD vs. TD, mean: 54.5% vs. 61.9%; Cohen’s d = 0.87, ηp2 = 0.14, P < 10–5). Overall preference for biological motion was significantly greater than the chance level (50%, equal looking time for both types of stimuli) in both groups of participants (both P-values < 10–8). Preference for biological motion was the only eye movement metric that was significantly related to age, with older individuals (ASD and TD) showing a decrease in preference with age (Supplementary Table 1) (Supplementary Fig. 2). Percentage of time the first fixation was on biological motion was similar between the two groups of participants (ASD vs. TD, mean: 54.2% vs. 52.7%; Cohen’s d = 0.25, ηp2 = 0.01, P = 0.26). In both groups of participants, percentage of time the first fixation was on biological motion significantly exceeded the chance level (in both groups P-value < 0.01). Individuals with ASD showed greater average latencies of the first fixation on both biological (ASD vs. TD, mean: 649.1 ms vs. 521.3 ms; Cohen’s d = 0.56, ηp2 = 0.05, P < 0.01) and non-biological motion (639.7 ms vs. 530.9 ms; Cohen’s d = 0.46, ηp2 = 0.04, P < 0.03), as compared to TD controls. There was no significant difference in average latency of the first fixation between biological and non-biological stimuli when data from each group were analyzed separately (both P-values > 0.94). Qualitatively similar results were observed when accounting for an interaction between participant’s age and group (the interaction term in ANCOVA’s: all five P-values > 0.24), a potential effect of site on the eye movement metrics (the effect of site in ANCOVA’s: all five P-values > 0.10), and the violations of the homogeneity of variance assumption in the analyses (Supplementary Tables 6 and 7). Furthermore, the effect of participant group on all five metrics persisted after removing potential outliers present in the data (Supplementary Table 1).
Restricting analyses to the groups of individuals with ASD with a specific level of IQ (low: n = 34 participants; average/normal: n = 63; high: n = 24) showed qualitatively similar results (Supplementary Table 2). Interestingly, as the IQ of the ASD group increased, “% total valid time” and average latency of the first fixation on either type of stimuli were more similar to those of the TD group. On the contrary, as the IQ of the ASD group increased, preference for biological motion deviated more from that observed in the TD group. Importantly, no systematic relationship between any of the five eye movement metrics and KBIT-2 IQ composite score was observed across the three tested IQ levels (Supplementary Table 3). Altogether, this rules out an explanation of the observed differences in the eye movement metrics between the two groups of participants by IQ.
Preference for Biological Motion Across Different Levels of ASD Symptom Severity
To test for effect of severity of ASD symptoms on preference for biological motion for each behavior rating scale and symptom, participants with ASD were ranked according to the numeric score of that symptom and then split into three similarly-sized, non-overlapping groups (mild, moderate, severe) (Supplementary Table 4). For all five caregiver-reported behavior rating scales and ADOS-2, all symptoms, and three severity groupings, mean preference for biological motion exceeded the chance level (50%), reaching statistical significance in 80 out of 87 (92%) comparisons (all P-values < 0.05) (Table 2). All non-significant comparisons were in the mild symptom severity category.
Relationship Between Eye Movement Metrics and ASD Symptoms
To assess relationships between eye movement metrics and different ASD symptoms, as assessed by five caregiver-reported behavior rating scales and ADOS-2, each metric (n = 5) was examined for correlation with each symptom numeric score (n = 29). Only 5 of 145 computed correlation coefficients (3.4%) reached statistical significance (Supplementary Table 5; Supplementary Figs. 4–8). The ADOS-2 “restricted and repetitive behavior” significantly and positively correlated with % total valid time (rS = 0.192, P < 0.04). Similarly, preference for biological motion correlated with the ABI “mental health” (rS = 0.185, P < 0.05). Lastly, average latency of the first fixation on non-biological motion significantly and negatively correlated with the ABC “stereotypic behavior” (rS = − 0.245, P < 0.01), the RBS-R “restricted behavior” (rS = − 0.192, P < 0.04) and “stereotyped behavior” (rS = -− 0.191, P < 0.04).
Discussion
We report differences in eye movements between individuals with ASD and TD controls while viewing biological motion. Overall, individuals with ASD paid less attention to the presented stimuli than TD controls. In comparison to TD controls, individuals with ASD spent less time looking at biological vs. non-biological motion, accounting for differences in total attention to stimuli. Nevertheless, individuals with ASD revealed a subtle preference for biological motion. In addition, both the ASD and TD groups showed a subtle preference to make their first fixation on biological motion, and this preference did not differ between the two groups. Moreover, individuals with ASD showed a greater average latency of the first fixation than TD controls regardless the type of presented stimuli.
Our results suggest that the reduced preference for biological motion observed in studies of younger children (Annaz et al. 2012; Klin et al. 2009; Wang et al. 2015) remain in older individuals with ASD. Differences between the ASD and TD groups in time spent looking at biological motion were persistent across phenotypic groups of varying severity as determined by behavior rating scales, suggesting that reduced preferences for biological motion were evident in individuals with ASD regardless of severity. Although the preference for biological motion over non-biological motion in individuals with ASD was less than in the TD group, our results consistently showed that across phenotypic groups of individuals with ASD this preference was still significantly greater than expected by chance. Individuals with ASD are still more likely to look at biological motion than non-biological motion, but less likely than the TD group. Interestingly, familiarization with the stimuli that could have been presumably built-up across stimulus presentations did not modulate preference for biological motion in either group of participants (see Supplementary Material). Importantly, effect sizes for preferences were small, which may explain the lack of consistency in previously reported findings, particularly in studies with small sample sizes.
Sifre et al. (2018) observed a U-shaped curve in the occurrence of biological motion preference in typically developing infants from neonates to 2 years, with no evidence of the preference seen in 2-month olds. They suggest that this observation is the result of a shift from experience-expectant to experience-dependent mechanisms that drive visual attention to biological motion, occurring at around 2 months of age. Our observation that, despite an overall preference for biological motion, a reduced preference in comparison to the TD group remains in older children and adults with ASD, which may be accounted for by differences in experience-dependent mechanisms in this group. For example, the Social Motivation Theory (Chevallier et al. 2012) suggests that less reward value is assigned to social stimuli, and this can be linked to motivation to engage socially and increased social impairments in ASD (Dawson et al. 2004). Reward value of biological motion specifically was established in a group of TD individuals (Williams and Cross 2018). Individuals in this group with more autistic traits showed reduced motivation for biological motion. Although we did not find that reduced preference for biological motion was related to any caregiver reported observation of social skills in the ASD group alone, the reduced preference observed in comparison to the TD group may reflect differences in reward values associated with biological motion between the groups. If so, it is possible that interventions that impact on reward values may lead to changes in biological motion preference that precede desired improvements in social motivation. In this way, increase in biological motion preference in ASD may serve as a biomarker for change in response to intervention.
There were a few relationships between any of the eye tracking metrics in ASD and behavioral reports using the behavioral rating scales. Given the modest size of these correlations, none of the observed relationships would remain if adjustments were made for multiple comparisons. Altogether, we believe that our data do not provide compelling evidence for the existence of a relationship between severity of ASD symptoms and preference for biological motion. Instead, these correlations should be interpreted with a great caution and rather be used to inform future research about the existence of potential links between behavioral reports and eye-tracking measures. Note, however, that the lack of correlations appears to be genuine given controls for participant’s age, gender and IQ as well as the size and heterogeneity of the ASD sample used to compute the correlations. Furthermore, the significant differences observed in the eye-tracking metrics between the two groups of participants suggest that these metrics convey information on ASD that can be orthogonal to that captured by behavior rating scales. An interesting observation was the relationship with IQ, that individuals with ASD who had a higher IQ spent less time looking at biological motion. It is possible that different mechanisms are driving attention to or away from biological motion across development in ASD. Contrary to our results, Rutherford and Troje (2012) found that perception of biological motion was increased with higher IQ in ASD. It is possible that individuals with ASD and higher IQ in the current study switched attention more quickly to non-biological motion following an initial fixation and perception of biological motion, thus leading to increased time spent on viewing non-biological motion compared to those with ASD and lower IQ. The relationship between social attention and IQ in ASD warrants further research. Besides the relationship with IQ, preference for biological motion tended to slightly decrease with age across both groups of participants. Though this relationship would not survive adjustment for multiple comparisons and disappeared after removing potential outliers present in the data, it may reflect change over time in cognitive processes that impact preference for biological motion as an individual matures. However, the latter proposal is challenged by the lack of effect of age on attention to social stimuli in the eye-tracking studies of ASD, as is reported in two recent meta-analysis studies by 2016 and Frazier et al. (2017).
Although latency to the first fixation was estimated without verifying participant’s attention on the center of the screen in the beginning of each video stimulus presentation, which could have had an impact on the estimates, this should not have influenced between-group comparisons. Longer latency to first fixation was observed in the ASD group, regardless of whether first fixation was to biological or non-biological motion. This suggests that allocation of visual attention may differ in ASD, regardless of the nature of the stimuli. For example, individuals with ASD may experience difficulties with disengagement and might be impacted by the use of a cartoon image in place of the cross hair in this task. Slower first fixation may also be the result of a more cognitively-driven approach at first fixation, and/or differences in motion sensitivity/perception. The current task was not developed to study these potential differences, and since features for first fixation were estimated irrespective of whether participants were looking at the screen between clip presentations, it may be that the reduced valid time viewing the screen could account for some of the delays in first fixation in ASD. However, observed differences in latency to first fixation warrant further investigation to understand the mechanisms that might impact allocation of visual attention in ASD.
Limitations
This study was part of a larger prospective, non-observational study designed to develop measures of change in ASD (Ness et al. 2019). One focus of the study was on obtaining quality data across a number of tasks and biosensors within the ASD population. Given the heterogeneity of this group, the aim was to maximize the collection of data from this sample. A group of TD individuals was added for comparison. As this was not the main focus of the study, this led to a smaller and less characterized TD group. In particular, the TD group did not have IQ measures, which limits inferences about the impact of IQ on performance. In addition, controls for multiple comparison were not performed in these analyses. The current results should be considered exploratory, and further validation in additional studies is required.
This study did not involve an intervention, and therefore the responsiveness to change of the features that discriminate between the ASD and TD groups could not be assessed, and this is a next step for an ongoing intervention study (NCT03664232). However, unlike Umbricht et al. (2017) who determined that first orientation to biological motion was less in an ASD group, and increased following vasopressin 1a receptor antagonist administration, we did not find evidence of between-group differences for this feature. Future studies are required to determine whether attention to biological motion can be increased in response to intervention, and whether this could be a precursor to other observable change in social interaction.
Summary and Conclusions
The examined group of individuals with ASD, aged 6 to adult, showed differences in visual attention to biological motion compared to TD individuals. This agrees with previously reported observations in younger children with ASD. The differences observed in this older group of individuals with ASD may also reflect differences in reward allocation of social stimuli and support the Social Motivation Theory. Though the revealed differences are small, they may contribute to observable social interaction difficulties in ASD over time. Therefore, differences in allocation of visual attention may be a risk marker or useful contributor to diagnostic criteria in ASD and could leverage monitoring outcomes in clinical trials. Nevertheless, future interventional studies are still needed to determine whether these group differences are sensitive to change in individuals with ASD.
Data Availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at https://yoda.yale.edu.
References
Aman, M. G., Novotny, S., Samango-Sprouse, C., Lecavalier, L., Leonard, E., Gadow, K. D., et al. (2004). Outcome measures for clinical drug trials in autism. CNS Spectrums, 9(1), 36–47.
Aman, M. G., & Singh, N. N. (2017). Aberrant behavior checklist manual (2nd ed.). East Aurora, NY: Slosson Educational Publications Inc.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders, 5th Edition: DSM-5. Arlinglton, TX: American Psychiatric Publishing.
Annaz, D., Campbell, R., Coleman, M., Milne, E., & Swettenham, J. (2012). Young children with autism spectrum disorder do not preferentially attend to biological motion. Journal of Autism and Developmental Disorders, 42(3), 401–408.
Bangerter, A., Chatterjee, M., Manfredonia, J., Manyakov, N. V., Ness, S., Boice, M. A., et al. (2020a). Automated recognition of spontaneous facial expression in individuals with autism spectrum disorder: Parsing response variability. Molecular Autism, 11(1), 31.
Bangerter, A., Chatterjee, M., Manyakov, N. V., Ness, S., Lewin, D., Skalkin, A., et al. (2020b). Relationship between sleep and behavior in autism spectrum disorder: Exploring the impact of sleep variability. Frontiers in Neuroscience, 14, 211.
Bangerter, A., Ness, S., Aman, M. G., Esbensen, A. J., Goodwin, M. S., Dawson, G., et al. (2017). Autism behavior inventory: A novel tool for assessing core and associated symptoms of autism spectrum disorder. Journal of Child and Adolescent Psychopharmacology, 27(9), 814–822.
Blake, R., & Shiffrar, M. (2007). Perception of human motion. Annual Review of Psychology, 58, 47–73.
Blignaut, P., & Wium, D. (2014). Eye-tracking data quality as affected by ethnicity and experimental design. Behavior Research Methods, 46(1), 67–80.
Bodfish, J., Symons, F., & Lewis, M. (1999). The Repetitive Behavior Scale (Western Carolina Center Research Reports). Morganton, NC: Western Carolina Center.
Bradshaw, J., Shic, F., Holden, A. N., Horowitz, E. J., Barrett, A. C., German, T. C., et al. (2019). The use of eye tracking as a biomarker of treatment outcome in a pilot randomized clinical trial for young children with autism. Autism Research, 12(5), 779–793.
Campbell, D. J., Shic, F., Macari, S., & Chawarska, K. (2014). Gaze response to dyadic bids at 2 years related to outcomes at 3 years in autism spectrum disorders: A subtyping analysis. Journal of Autism and Developmental Disorders, 44(2), 431–442.
Chawarska, K., Macari, S., & Shic, F. (2012). Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry, 53(8), 903–913.
Chawarska, K., Ye, S., Shic, F., & Chen, L. (2016). Multilevel differences in spontaneous social attention in toddlers with autism spectrum disorder. Child Development, 87(2), 543–557.
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S., & Schultz, R. T. (2012). The social motivation theory of autism. Trends in Cognitive Sciences, 16(4), 231–239.
Chita-Tegmark, M. (2016). Social attention in ASD: A review and meta-analysis of eye-tracking studies. Research in Developmental Disabilities, 48, 79–93.
CMU Graphics Lab. (2011). Motion Capture Database. Retrieved October 22, 2019 from https://mocap.cs.cmu.edu.
Constantino, J. N., Davis, S. A., Todd, R. D., Schindler, M. K., Gross, M. M., Brophy, S. L., et al. (2003). Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders, 33(4), 427–433.
Dawson, G., Toth, K., Abbott, R., Osterling, J., Munson, J., Estes, A., et al. (2004). Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology, 40(2), 271–283.
Dawson, G., Bernier, R., & Ring, R. H. (2012). Social attention: A possible early indicator of efficacy in autism clinical trials. Journal of Neurodevelopmental Disorders, 4(1), 11.
Franchini, M., Wood de Wilde, H., Glaser, B., Gentaz, E., Eliez, S., & Schaer, M. (2016). Brief report: A preference for biological motion predicts a reduction in symptom severity 1 year later in preschoolers with autism spectrum disorders. Frontiers in Psychiatry, 7, 143.
Frazier, T. W., Strauss, M., Klingemier, E. W., Zetzer, E. E., Hardan, A. Y., Eng, C., et al. (2017). A meta-analysis of gaze differences to social and nonsocial information between individuals with and without autism. Journal of the American Academy of Child and Adolescent Psychiatry, 56(7), 546–555.
Fujioka, T., Inohara, K., Okamoto, Y., Masuya, Y., Ishitobi, M., Saito, D. N., et al. (2016). Gazefinder as a clinical supplementary tool for discriminating between autism spectrum disorder and typical development in male adolescents and adults. Molecular Autism, 7, 19.
Fujisawa, T. X., Tanaka, S., Saito, D. N., Kosaka, H., & Tomoda, A. (2014). Visual attention for social information and salivary oxytocin levels in preschool children with autism spectrum disorders: An eye-tracking study. Frontiers in Neuroscience, 8, 295.
Gadow, K. D., & Sprafkin, J. (1997). Adolescent Symptom Inventory 4 Screening Manual. Stony Brook, NY: Checkmate Plus LTD.
Guillon, Q., Hadjikhani, N., Baduel, S., & Rogé, B. (2014). Visual social attention in autism spectrum disorder: Insights from eye tracking studies. Neuroscience and Biobehavioral Reviews, 42, 279–297.
Hergueta, T., Baker, R., & Dunbar, G. C. (1998). The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(Suppl 20), 22–33.
Jagannatha, S., Sargsyan, D., Manyakov, N. V., Skalkin, A., Bangerter, A., Ness, S., et al. (2019). A practical application of data mining methods to build predictive models for autism spectrum disorder based on biosensor data from Janssen Autism Knowledge Engine (JAKE®). Statistics in Biopharmaceutical Research. https://doi.org/10.1080/19466315.2018.1527247.
Johansson, G. (1973). Visual perception of biological motion and a model for its analysis. Perception and Psychophysics, 14(2), 201–211.
Johnson, M. H. (2006). Biological motion: A perceptual life detector? Current Biology, 16(10), 376–377.
Kaiser, M. D., Delmolino, L., Tanaka, J. W., & Shiffrar, M. (2010). Comparison of visual sensitivity to human and object motion in autism spectrum disorder. Autism Research, 3(4), 191–195.
Kaufman, A. S., & Kaufman, N. L. (2004). Kaufman Brief Intelligence Test. Bloomington, MN: Pearson Inc.
Keehn, B., Müller, R. A., & Townsend, J. (2013). Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews, 37(2), 164–183.
Kéri, S., & Benedek, G. (2009). Oxytocin enhances the perception of biological motion in humans. Cognitive, Affective, & Behavioral Neuroscience, 9(3), 237–241.
Kim, S. (2015). ppcor: An R package for a fast calculation to semi-partial correlation coefficients. Communications for Statistical Applications and Methods, 22(6), 665–674.
Klin, A., Lin, D. J., Gorrindo, P., Ramsay, G., & Jones, W. (2009). Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature, 459(7244), 257–261.
Klin, A. (2018). Biomarkers in autism spectrum disorder: Challenges, advances, and the need for biomarkers of relevance to public health. Focus, 16(2), 135–142.
Landry, R., & Bryson, S. E. (2004). Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry, 45(6), 1115–1122.
Lord, C., Luyster, R., Gotham, K., & Guthrie, W. (2012). Autism Diagnostic Observation Schedule (2nd ed.). Torrence, CA: Western Psychological Services.
Manfredonia, J., Bangerter, A., Manyakov, N. V., Ness, S., Lewin, D., Skalkin, A., et al. (2018). Automatic recognition of posed facial expression of emotion in individuals with autism spectrum disorder. Journal of Autism and Developmental Disorders, 49(1), 279–293.
Manyakov, N. V., Bangerter, A., Chatterjee, M., Mason, L., Ness, S., Lewin, D., et al. (2018). visual exploration in autism spectrum disorder: Exploring age differences and dynamic features using recurrence quantification analysis. Autism Research, 11(11), 1554–1566.
McPartland, J. C. (2017). Developing clinically practicable biomarkers for autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(9), 2935–2937.
Murias, M., Major, S., Davlantis, K., Franz, L., Harris, A., Rardin, B., et al. (2018). Validation of eye-tracking measures of social attention as a potential biomarker for autism clinical trials. Autism Research, 11(1), 166–174.
Ness, S. L., Bangerter, A., Manyakov, N. V., Lewin, D., Boice, M., Skalkin, A., et al. (2019). An observational study with The Janssen Autism Knowledge Engine (JAKE®) in individuals with autism spectrum disorder. Frontiers in Neuroscience, 13, 111.
Perry, A., Bentin, S., Shalev, I., Israel, S., Uzefovsky, F., Bar-On, D., et al. (2010). Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology, 35(10), 1446–1453.
Pierce, K., Conant, D., Hazin, R., Stoner, R., & Desmond, J. (2011). Preference for geometric patterns early in life as a risk factor for autism. Archives of General Psychiatry, 68(1), 101–109.
Puglia, M. H., & Morris, J. P. (2017). Neural response to biological motion in healthy adults varies as a function of autistic-like traits. Frontiers in Neuroscience, 11, 404.
Rutherford, M., & Troje, N. F. (2012). IQ predicts biological motion perception in autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(4), 557–565.
Rutter, M., Bailey, A., & Lord, C. (2003). Social Communication Questionnaire: SCQ (W-381). Los Angeles, CA: Western Psychological Services.
Sacrey, L. A., Armstrong, V. L., Bryson, S. E., & Zwaigenbaum, L. (2014). Impairments to visual disengagement in autism spectrum disorder: A review of experimental studies from infancy to adulthood. Neuroscience and Biobehavioral Reviews, 47, 559–577.
Sargsyan, D., Jagannatha, S., Manyakov, N. V., Skalkin, A., Bangerter, A., Ness, S., et al. (2019). Feature selection with weighted importance index in an autism spectrum disorder study. Statistics in Biopharmaceutical Research. https://doi.org/10.1080/19466315.2018.1537886.
Sasson, N. J., Turner-Brown, L. M., Holtzclaw, T. N., Lam, K. S., & Bodfish, J. W. (2008). Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research, 1(1), 31–42.
Sasson, N. J., Elison, J. T., Turner-Brown, L. M., Dichter, G. S., & Bodfish, J. W. (2011). Brief report: Circumscribed attention in young children with autism. Journal of Autism and Developmental Disorders, 41(2), 242–247.
Shic, F., Bradshaw, J., Klin, A., Scassellati, B., & Chawarska, K. (2011). Limited activity monitoring in toddlers with autism spectrum disorder. Brain Research, 1380, 246–254.
Shic, F., Macari, S., & Chawarska, K. (2014). Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder. Biological Psychiatry, 75(3), 231–237.
Sheehan, D. V., Sheehan, K. H., Shytle, R. D., Janavs, J., Bannon, Y., Rogers, J. E., et al. (2010). Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID). The Journal of Clinical Psychiatry, 71(3), 313–326.
Sifre, R., Olson, L., Gillespie, S., Klin, A., Jones, W., & Shultz, S. (2018). A longitudinal investigation of preferential attention to biological motion in 2- to 24-month-old infants. Scientific Reports, 8(1), 2527. https://doi.org/10.1038/s41598-018-20808-0.
Simion, F., Regolin, L., & Bulf, H. (2008). A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences, 105(2), 809–813.
Umbricht, D., del Valle Rubido, M., Hollander, E., McCracken, J. T., Shic, F., Scahill, L., et al. (2017). A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology, 42(9), 1924.
van der Lans, R., Wedel, M., & Pieters, R. (2011). Defining eye-fixation sequences across individuals and tasks: The Binocular-Individual Threshold (BIT) algorithm. Behavior Research Methods, 43(1), 239–257.
Wang, L.-H., Chien, S. H.-L., Hu, S.-F., Chen, T.-Y., & Chen, H.-S. (2015). Children with autism spectrum disorders are less proficient in action identification and lacking a preference for upright point-light biological motion displays. Research in Autism Spectrum Disorders, 11, 63–76.
Wang, Y., Wang, L., Xu, Q., Liu, D., Chen, L., Troje, N. F., et al. (2018). Heritable aspects of biological motion perception and its covariation with autistic traits. Proceedings of the National Academy of Sciences, 115(8), 1937–1942.
Williams, E. H., & Cross, E. S. (2018). Decreased reward value of biological motion among individuals with autistic traits. Cognition, 171, 1–9.
Acknowledgments
The authors thank the study participants and the following investigators for their participation in this study: Arizona: Christopher J. Smith, PhD; California: Bennett Leventhal, MD and Robert Hendren; Connecticut (at the time of study conduct): Frederick Shic, PhD; Massachusetts: Jean Frazier, MD; New Jersey: Yvette Janvier, MD; New York: Russell Tobe, MD; North Carolina: Geraldine Dawson, PhD; Pennsylvania: Judith S. Miller, PhD; Washington: Bryan King, MD. Stacey E. Shehin, PhD, (PRA Health Sciences) provided medical writing assistance, which was funded by Janssen Research & Development, LLC, and Ellen Baum, PhD (Janssen Global Services) provided additional editorial support. Portions of this study have been previously presented at the 18th Annual Meeting of the International Society for Autism Research (INSAR), May 1-4, 2019, Montreal, QC, Canada. This study was funded by Janssen Research & Development, LLC.
Funding
This study was funded by Janssen Research & Development, LLC.
Author information
Authors and Affiliations
Contributions
DAK, NVM, AB, SN, AS, MB, MSG, GD, RH, BL, FS and GP contributed to the study design and data collection. All authors were involved in data analysis and interpretation. All authors had full access to all the data in the study and take responsibility for integrity of the data and the accuracy of the data analyses. All authors meet ICMJE criteria and all those who fulfilled those criteria are listed as authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
D.A.K., N.V.M are employees of Janssen Pharmaceutica NV and may hold company equity. A.B., S.N., M.B. and G.P. are employees of Janssen Research & Development, LLC, and may hold company equity. At the time of study conduct, A.S. was an employee of Janssen Research & Development, LLC, and could hold company equity. M.S.G. has received research and consulting funding from Janssen Research & Development. G.D. is on the Scientific Advisory Boards of Janssen Research & Development, LLC, Akili, Inc., LabCorp, Inc., and Roche Pharmaceutical Company, a consultant for Apple, Inc., Gerson Lehrman Group, and Axial Ventures, has received grant funding from Janssen Research and Development, LLC, and is CEO of DASIO, LLC. She receives royalties from Guilford Press, Springer, and Oxford University Press. R.H. received reimbursement for consultation from Janssen Research & Development, LLC. B.L. has received research grant funding from the NIH, is a consultant to Janssen Research & Development, LLC, the Illinois Children’s Healthcare Foundation, and is a board member of the Brain Research Foundation. F.S. is on the Scientific Advisory Board, is a consultant to, and received grant funding from Janssen Research & Development, LLC, and has also received grant funding from Roche.
Ethical Approval
Institutional Review Boards approved the study protocol and amendments. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, consistent with Good Clinical Practices and applicable regulatory requirements.
Informed Consent
Participants, their parents (for participants < 18 years old), or legally authorized representatives provided written informed consent before joining the study. Participants also provided assent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaliukhovich, D.A., Manyakov, N.V., Bangerter, A. et al. Visual Preference for Biological Motion in Children and Adults with Autism Spectrum Disorder: An Eye-Tracking Study. J Autism Dev Disord 51, 2369–2380 (2021). https://doi.org/10.1007/s10803-020-04707-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-020-04707-w