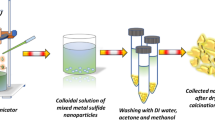
Abstract
Hybrid supercapacitors are energy storage technology offering higher power and energy density as compared to capacitors and batteries. Cobalt-doped manganese oxide (Co@MnO2) was synthesized using an easy and affordable sol–gel process and measured the electrochemical properties. A value of the specific capacity of 1141.42 Cg−1 was obtained which was larger in comparison to the reference sample (MnO2 = 673.79 Cg−1). The value of the specific capacitance was achieved 1902 Fg−1. To design a hybrid supercapacitor device, Co@MnO2 was used as the positive electrode and the activated carbon was employed as the negative electrode in two-electrode assembly. According to calculations, the measured value of the specific capacitance of Co@MnO2 was 713.25 Fg−1. The charge storage mechanism is supported with the help of Randles–Ševčík and Dunn’s models. The estimated value of energy and power densities were 3200 Wh kg−1 and 24 Wkg−1, respectively. The stability of this device was checked by putting it to 1000 charging and discharging cycles, and it retained 86% of its initial capacity. Our result provides a platform for enhancing the functionality of energy storage systems.
Graphical abstract








Similar content being viewed by others
Data availability
The data are available on request.
References
Zuo W et al (2017) Battery-supercapacitor hybrid devices: recent progress and future prospects. Adv Sci 4(7):1600539
Yu Z et al (2015) Supercapacitor electrode materials: nanostructures from 0 to 3 dimensions. Energy Environ Sci 8(3):702–730
Barnett S, Irvine J, Vohs J (2004) Advanced anodes for high-temperature fuel cells. Nat Mater 3(1):17–27
Lu Q et al (2011) Supercapacitor electrodes with high-energy and power densities prepared from monolithic NiO/Ni nanocomposites. Angew Chem Int Ed 50(30):6847–6850
Omar FS et al (2017) A promising binary nanocomposite of zinc cobaltite intercalated with polyaniline for supercapacitor and hydrazine sensor. J Alloys Compd 716:96–105
Gupta V, Miura N (2006) Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim Acta 52(4):1721–1726
Rahmanifar MS et al (2019) Asymmetric supercapacitors: an alternative to activated carbon negative electrodes based on earth abundant elements. Mater Today Energy 12:26–36
Iqbal J et al (2019) Density functional theory simulation of cobalt oxide aggregation and facile synthesis of a cobalt oxide, gold and multiwalled carbon nanotube based ternary composite for a high performance supercapattery. New J Chem 43(33):13183–13195
Pandolfo AG, Hollenkamp AF (2006) Carbon properties and their role in supercapacitors. J Power Sources 157(1):11–27
Yusin S, Bannov A (2017) Synthesis of composite electrodes for supercapacitors based on carbon materials and the metal oxide/metal hydroxide system. Prot Met Phys Chem Surf 53(3):475–482
Sato T, Masuda G, Takagi K (2004) Electrochemical properties of novel ionic liquids for electric double layer capacitor applications. Electrochim Acta 49(21):3603–3611
Han S et al (2019) Mechanical and electrical properties of graphene and carbon nanotube reinforced epoxy adhesives: experimental and numerical analysis. Compos Part A: Appl Sci Manufac 120:116–126
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4(4):217–224
Iqbal MZ et al (2020) Strontium phosphide-polyaniline composites for high performance supercapattery devices. Ceram Int 46(8):10203–10214
Shahabuddin S et al (2019) Polyaniline-SrTiO3 nanocube based binary nanocomposite as highly stable electrode material for high performance supercapaterry. Ceram Int 45(9):11428–11437
Simon P, Gogotsi Y, Peter R (2010) Materials for electrochemical capacitors in nanoscience and technology. Nat Rev Mater. https://doi.org/10.1038/nmat2297
Wang D et al (2009) Self-assembled TiO2–graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano 3(4):907–914
Li L et al (2010) Synthesis and electrochemical properties of two types of highly ordered mesoporous MnO2. Electrochim Acta 55(5):1682–1686
Cheng F et al (2006) Facile controlled synthesis of MnO2 nanostructures of novel shapes and their application in batteries. Inorg Chem 45(5):2038–2044
Li Z et al (2011) Synthesis of hydrothermally reduced graphene/MnO2 composites and their electrochemical properties as supercapacitors. J Power Sources 196(19):8160–8165
Chabre Y, Pannetier J (1995) Structural and electrochemical properties of the proton/γ-MnO2 system. Prog Solid State Chem 23(1):1–130
Wolfenstine J, Allen J (2004) LiNiPO4–LiCoPO4 solid solutions as cathodes. J Power Sources 136(1):150–153
Minakshi M et al (2011) Synthesis and characterization of olivine LiNiPO4 for aqueous rechargeable battery. Electrochim Acta 56(11):4356–4360
Li Z et al (2011) Flexible graphene/MnO 2 composite papers for supercapacitor electrodes. J Mater Chem 21(38):14706–14711
Iqbal MZ et al (2020) Cobalt-oxide/carbon composites for asymmetric solid-state supercapacitors. Mater Res Bull 131:110974
Kalu E et al (2001) Cyclic voltammetric studies of the effects of time and temperature on the capacitance of electrochemically deposited nickel hydroxide. J Power Sources 92(1–2):163–167
Yin B-S et al (2016) In situ growth of free-standing all metal oxide asymmetric supercapacitor. ACS Appl Mater Interfaces 8(39):26019–26029
Sun J et al (2017) Recent progress of fiber-shaped asymmetric supercapacitors. Mater Today Energy 5:1–14
Zhi M et al (2013) Nanostructured carbon–metal oxide composite electrodes for supercapacitors: a review. Nanoscale 5(1):72–88
Shao Y et al (2016) Three-dimensional hierarchical ni x Co1–x O/Ni y Co2–y P@ C hybrids on nickel foam for excellent supercapacitors. ACS Appl Mater Interfaces 8(51):35368–35376
Li T, Kaercher S, Roesky PW (2014) Synthesis, structure and reactivity of rare-earth metal complexes containing anionic phosphorus ligands. Chem Soc Rev 43(1):42–57
Xia X et al (2011) Three-dimentional porous nano-Ni/Co (OH) 2 nanoflake composite film: a pseudocapacitive material with superior performance. J Phys Chem C 115(45):22662–22668
Xiao W et al (2010) Synthesis, characterization, and lithium storage capability of AMoO4 (A = Ni, Co) nanorods. Chem Mater 22(3):746–754
Park K-S et al (2012) Electrochemical performance of Ni x Co 1-x MoO 4 (0 ≤ x ≤ 1) nanowire anodes for lithium-ion batteries. Nanoscale Res Lett 7:1–7
Xiao K et al (2015) Honeycomb-like NiMoO 4 ultrathin nanosheet arrays for high-performance electrochemical energy storage. J Mater Chem A 3(11):6128–6135
Sharma P et al (2020) Zn Metal Atom Doping on the Surface plane of One-Dimesional NiMoO4 nanorods with Improved Redox Chemistry. ACS Appl Mater Interfaces 12(40):44815–44829
Minakshi M et al (2018) New insights into the electrochemistry of magnesium molybdate hierarchical architectures for high performance sodium devices. Nanoscale 10(27):13277–13288
Iqbal MZ et al (2020) Role of graphene and transition metal dichalcogenides as hole transport layer and counter electrode in solar cells. Int J Energy Res 44(3):1464–1487
Qian Y, Du J, Kang DJ (2019) Enhanced electrochemical performance of porous co-doped TiO2 nanomaterials prepared by a solvothermal method. Microporous Mesoporous Mater 273:148–155
Srikesh G, Nesaraj AS (2022) Facile soft chemical synthesis and characterisation of novel cobalt doped nickel oxide based nanostructured electrode materials for electrochemical capacitors. Mater Technol 37(3):190–203
Sudhakar Y, Cortiñas SM, Selvakumar M (2019) Sequential layer-by-layer engineered polypyrrole-activated carbon multilayer films: high-energy composite electrode materials for symmetrical supercapacitors. Mater Technol 34(3):126–134
Wickramaarachchi K, Minakshi M (2022) Consequences of electrodeposition parameters on the microstructure and electrochemical behavior of electrolytic manganese dioxide (EMD) for supercapacitor. Ceram Int 48(14):19913–19924
Magar HS, Hassan RY, Mulchandani A (2021) Electrochemical impedance spectroscopy (EIS): principles, construction, and biosensing applications. Sensors 21(19):6578
Ehsan MA, Hakeem AS, Rehman A (2020) Hierarchical growth of CoO nanoflower thin films influencing the electrocatalytic oxygen evolution reaction. Electrocatalysis 11(3):282–291
Jin M et al (2017) Different distribution of in-situ thin carbon layer in hollow cobalt sulfide nanocages and their application for supercapacitors. J Power Sources 341:294–301
Simon P, Gogotsi Y, Dunn B (2014) Where do batteries end and supercapacitors begin? Science 343(6176):1210–1211
Iqbal MF et al (2018) Excellent electrochemical performance of graphene oxide based strontium sulfide nanorods for supercapacitor applications. Electrochim Acta 273:136–144
Iqbal MZ et al (2020) Co-MOF/polyaniline-based electrode material for high performance supercapattery devices. Electrochim Acta 346:136039
Iqbal MZ et al (2020) Hydrothermally synthesized zinc phosphate-rGO composites for supercapattery devices. J Electroanal Chem 871:114299
Iqbal MF et al (2017) High specific capacitance and energy density of synthesized graphene oxide based hierarchical Al2S3 nanorambutan for supercapacitor applications. Electrochim Acta 246:1097–1103
Shameem A et al (2019) Electrochemical performance and optimization of α-NiMoO 4 by different facile synthetic approach for supercapacitor application. J Mater Sci: Mater Electron 30:3305–3315
Hoque M (2013) The oxygen reduction reaction in non- aqueous electrolytes: li-air battery applications.
Jadhav SM et al (2021) Cobalt-doped Manganese Dioxide Hierarchical Nanostructures for Enhancing Pseudocapacitive Properties. ACS Omega 6(8):5717–5729
Lan Y et al (2018) Phosphorization boosts the capacitance of mixed metal nanosheet arrays for high performance supercapacitor electrodes. Nanoscale 10(25):11775–11781
Kashale AA et al (2019) Biosynthesized co-doped TiO2 nanoparticles based anode for lithium-ion battery application and investigating the influence of dopant concentrations on its performance. Compos Part B: Eng 167:44–50
Dhole I et al (2018) Optimization of techno-economic cobalt doped nickel oxide electrode designed for energy storage. in AIP Conference Proceedings. AIP Publishing LLC
Liu L et al (2019) Co doped α-Ni(OH)2 multiple-dimensional structure electrode material. Electrochim Acta 295:340–346
Xue C-F et al (2022) Magnesium oxide scaffolded preparation of N, O self-doped biochar with super-hydrophilic surface for aqueous supercapacitor with desired energy density. J Energy Storage 53:105193
Kim CH, Kim B-H (2015) Zinc oxide/activated carbon nanofiber composites for high-performance supercapacitor electrodes. J Power Sources 274:512–520
Lorkit P, Panapoy M, Ksapabutr B (2014) Iron Oxide-based Supercapacitor from Ferratrane Precursor via Sol–gel-hydrothermal process. Energy Procedia 56:466–473
Acknowledgements
The work was supported by Researchers Supporting Project number (RSP2023R492), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
AMA, MR, and NM worked on the experiment, data collection, analysis, interpretation of results, and writing the manuscript. MWI, GD, S.M, EAA, AM, and SME helped with the calculation process, performed the experiments, and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
It is confirmed that the submitted work should be original and should not have been published elsewhere in any form or language.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afzal, A.M., Muzaffar, N., Iqbal, M.W. et al. Exploring the charge storage mechanism in high-performance Co@MnO2-based hybrid supercapacitors using Randles–Ševčík and Dunn’s models. J Appl Electrochem 54, 65–76 (2024). https://doi.org/10.1007/s10800-023-01939-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01939-3