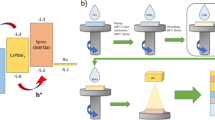
Abstract
Solution-processed, NiO thin films have been synthesized via spin coating technique. Nickel nitrate hexahydrate and 2-Aminoethanol have been used as nickel precursor and oxidizing agent, respectively for depositing layers of NiO solution on the FTO substrate. Investigating the effect of NiO layers on morphological, structural, electrochromic properties has been observed. An X-Ray diffraction study has confirmed the deposition of cubic phase nanostructured NiO with an observed average crystallite size varying from 15 to 25 nm. FESEM images of NiO thin films show the formation of nanorods and nano pebbles structures. Synthesized NiO thin films exhibit anodic electrochromism in 1 M KOH electrolyte solution, i.e., transition from bleached to coloration state due to intercalation of OH− ions and electrons with a switching time of around 1.2 s. Transportation of OH− ions and electrons at the interface of NiO thin film has been well explained using cyclic voltammetry, electrochemical impedance spectroscopy in potential window of −1.0 V to + 1.0 V. 20 layers of NiO thin film shows better electrochromic reversibility and stability. Also, it gives a Power conversion efficiency of 0.41% when used as hole transporting layer (HTL) in Dye sensitized solar cell (DSSC) application with maximum value of Jsc as 5.81 mA/cm2. Thus, synthesized NiO thin film has been a potential candidate for DSSC and electrochromic based applications.
Graphical abstract



















Similar content being viewed by others
References
Haque MA, Sheikh AD, Guan X, Wu T (2017) Metal oxides as efficient charge transporters in perovskite solar cells. Adv Energy Mater 7:1–23. https://doi.org/10.1002/aenm.201602803
Sk M, Yue CY, Ghosh K, Jena RK (2016) Review on advances in porous nanostructured nickel oxides and their composite electrodes for high-performance supercapacitors. J Power Sources 308:121–140. https://doi.org/10.1016/j.jpowsour.2016.01.056
Leilei T, Quanchao Z, Jia LI, Yueli SHI, Jianpeng C, Feng LU, Shigang SUN (2011) Mechanism of intercalation and deintercalation of lithium ions in graphene nanosheets. Chin Sci Bull 56:3204–3212. https://doi.org/10.1007/s11434-011-4609-6
Qi W, Gao Z (2015) Metal oxide nanoparticles in electroanalysis. Electroanalysis 27(9):2074–2090. https://doi.org/10.1002/elan.201500024
Liu N, Li J, Ma W, Liu W, Shi Y, Tao J, Zhang X, Su J, Li L, Gao Y (2014) Ultrathin and lightweight 3D free-standing Ni@Nio nanowire membrane electrode for a supercapacitor with excellent capacitance retention at high rates. ACS Appl Mater Interfaces 6:13627–13634. https://doi.org/10.1021/am503108x
Patil PS, Patil PR, Kamble SS, Pawar SH (2000) Thickness-dependent electrochromic properties of solution thermolyzed tungsten oxide thin. Sol Energy Mater Sol Cells 60(2):143–153. https://doi.org/10.1016/S0927-0248(99)00079-3
Kumar KN, Shaik H, Pawar A, Chandrashekar LN, Abdul S, Nithya G, Jafri RI, Madhavi V, Gupta J, Reddy GVA (2022) Effect of annealing and oxygen partial pressure on the RF sputtered WO3 thin films for electrochromic applications. Mater Today Proc 59:339–344. https://doi.org/10.1016/j.matpr.2021.11.185
Kumar KN, Shaik H, Chandrashekar LN, Aishwarya P, Abdul S, Nithya G, Madhavi V, Jafri RI, Gupta J, Reddy GVA (2022) On ion transport during the electrochemical reaction on plane and GLAD deposited WO3 thin films. Mater Today Proc 59:275–282. https://doi.org/10.1016/j.matpr.2021.11.113
Kumar KN, Nithya G, Shaik H, Hemanth B, Chethana M, Kishore K, Madhavi V, Jafri RI, Abdul S, Gupta J, Reddy GVA (2022) Simulation and fabrication of tungsten oxide thin films for electrochromic applications. Phys B Phys Condens Matter 640:413932. https://doi.org/10.1016/j.physb.2022.413932
Kumar KN, Shaik H, Gupta J, Abdul S, Jafri I, Pawar A, Madhavi V, Reddy AGV, Nithya G (2022) Sputter deposited tungsten oxide thin films and nanopillars: electrochromic perspective. Mater Chem Phys 278:125706. https://doi.org/10.1016/j.matchemphys.2022.125706
Mujawar SH, Inamdar AI, Patil SB, Patil PS (2006) Electrochromic properties of spray-deposited niobium oxide thin films. Solid State Ionics 177:3333–3338. https://doi.org/10.1016/j.ssi.2006.08.032
Paulose R, Mohan R, Parihar V (2017) Nanostructured nickel oxide and its electrochemical behaviour—a brief review. Nano-Struct Nano-Objects 11:102–111. https://doi.org/10.1016/j.nanoso.2017.07.003
Sun DL, Zhao BW, Liu JB, Wang H, Yan H (2017) Application of nickel oxide nanoparticles in electrochromic materials. Ionics (Kiel) 23:1509–1515. https://doi.org/10.1007/s11581-017-1974-4
Zhang W, Li H, Hopmann E, Elezzabi AY (2020) Nanostructured inorganic electrochromic materials for light applications. Nanophotonics 10(2):825–850. https://doi.org/10.1515/nanoph-2020-0474
Nail BA, Fields JM, Zhao J, Wang J, Greaney MJ, Brutchey RL, Osterloh FE (2015) Nickel oxide particles catalyze photochemical hydrogen evolution from water-nanoscaling promotes p—type character and minority carrier extraction. ACS Nano 9(5):5135–5142. https://doi.org/10.1021/acsnano.5b00435
Khalil A, Lalia BS, Hashaikeh R (2016) Nickel oxide nanocrystals as a lithium-ion battery anode: structure-performance relationship. J Mater Sci 51:6624–6638. https://doi.org/10.1007/s10853-016-9946-z
Wang B, Chen JS, Wang Z, Madhavi S, Wen X, Lou D (2012) Green synthesis of NiO nanobelts with exceptional pseudo-capacitive properties. Adv Energy Mater 2(10):1188–1192. https://doi.org/10.1002/aenm.201200008
Luyo C, Ionescu R, Reyes LF, Topalian Z, Estrada W, Llobet E, Granqvist CG, Heszler P (2009) Chemical gas sensing response of NiO nanoparticle films made by reactive gas deposition. Sens Actuat B 138:14–20. https://doi.org/10.1016/j.snb.2008.11.057
Wen R, Granqvist CG, Niklasson GA (2015) Anodic electrochromism for energy-efficient windows: cation/anion-based surface processes and effects of crystal facets in nickel oxide thin films. Adv Func Mater 25(22):3359–3370. https://doi.org/10.1002/adfm.201500676
Wang L, Hao Y, Zhao Y, Lai Q, Xu X (2010) Hydrothermal synthesis and electrochemical performance of NiO microspheres with different nanoscale building blocks. J Solid State Chem 183:2576–2581. https://doi.org/10.1016/j.jssc.2010.09.006
Nanoscale C, Meher SK, Justin P, Rao GR (2011) Nanoscale morphology dependent pseudocapacitance of NiO: influence of intercalating anions during synthesis. Nanoscale 3:683–692. https://doi.org/10.1039/c0nr00555j
Kim S, Lee J, Ahn H, Song H, Jang J (2013) Facile route to an efficient NiO supercapacitor with a three- dimensional nanonetwork morphology. ACS Appl Mater Inter 5:1595–1603. https://doi.org/10.1021/am3021894
Zhu J, Jiang J, Liu J, Ding R, Ding H, Feng Y, Wei G, Huang X (2011) Direct synthesis of porous NiO nanowall arrays on conductive substrates for supercapacitor application. J Solid State Chem 184:578–583. https://doi.org/10.1016/j.jssc.2011.01.019
Zhong J, Wang XL, Xia XH, Gu CD, Xiang JY, Zhang J, Tu JP (2011) Self-assembled sandwich-like NiO film and its application for Li-ion batteries. J Alloys Compd 509:3889–3893. https://doi.org/10.1016/j.jallcom.2010.12.151
Khairy M, El-safty SA (2014) Chemical Nanosized rambutan-like nickel oxides as electrochemical sensor and pseudocapacitor. Sens Actuat B Chem 193:644–652. https://doi.org/10.1016/j.snb.2013.11.113
Kalam A, Al-Shihri AS, Al-Sehemi AG, Awwad NS, Du G, Ahmad T (2013) Effect of pH on solvothermal synthesis of β-Ni(OH)2 and NiO nano-architectures: surface area studies, optical properties and adsorption studies. Superlattices Microstruct 55:83–97. https://doi.org/10.1016/j.spmi.2012.11.024
Kumar KN, Shaik H, Madhavi V et al (2022) Glancing angle sputter deposited tungsten trioxide (WO3) thin films for electrochromic applications. Appl Phys A 128:985. https://doi.org/10.1007/s00339-022-06124-5
Purushothaman KK, Muralidharan G (2009) The effect of annealing temperature on the electrochromic properties of nanostructured NiO films. Sol Energy Mater Sol Cells 93:1195–1201. https://doi.org/10.1016/j.solmat.2008.12.029
Vijayakumar S, Nagamuthu S, Muralidharan G (2013) Supercapacitor studies on NiO nano flakes synthesized through a microwave route. ACS Appl Mater Interface 5(6):2188–2196. https://doi.org/10.1021/am400012h
Vidales-Hurtado MA, Mendoza-Galván A (2008) Optical and structural characterization of nickel oxide-based thin films obtained by chemical bath deposition. Mater Chem Phys 107(1):33–38. https://doi.org/10.1016/j.matchemphys.2007.06.036
Sahu DR, Wu TJ, Wang SC, Huang JL (2017) Electrochromic behavior of NiO film prepared by e-beam evaporation. J Sci Adv Mater Devices 2:225–232. https://doi.org/10.1016/j.jsamd.2017.05.001
Uplane MM, Mujawar SH, Inamdar AI, Shinde PS, Sonavane AC, Patil PS (2007) Structural, optical and electrochromic properties of nickel oxide thin films grown from electrodeposited nickel sulphide. Appl Surf Sci 253(24):9365–9371. https://doi.org/10.1016/j.apsusc.2007.05.069
Xia XH, Tu JP, Zhang J, Wang XL, Zhang WK, Huang H (2008) Morphology effect on the electrochromic and electrochemical performances of NiO thin films. Electrochim Acta 53:5721–5724. https://doi.org/10.1016/j.electacta.2008.03.047
Kamil AF, Abdullah HI, Rheima AM, Mohammed SH (2021) Photochemical synthesized NiO nanoparticles based dye-sensitized solar cells: a comparative study on the counter electrodes and dye-sensitized concentrations. J Ovonic Res 17(3):299–305
Dalavi DS, Suryavanshi MJ, Patil DS, Mali SS, Moholkar AV, Kalagi SS, Vanalkar SA, Kang SR, Kim JH, Patil PS (2011) Nanoporous nickel oxide thin films and its improved electrochromic performance: effect of thickness. Appl Surf Sci 257(7):2647–2656. https://doi.org/10.1016/j.apsusc.2010.10.037
Sonavane AC, Inamdar AI, Shinde PS, Deshmukh HP, Patil RS, Patil PS (2010) Efficient electrochromic nickel oxide thin films by electrodeposition. J Alloys Compd 489:667–673. https://doi.org/10.1016/j.jallcom.2009.09.146
Sialvi MZ, Mortimer RJ, Wilcox GD, Teridi AM, Varley TS, Wijayantha KGU, Kirk CA (2013) Electrochromic and colorimetric properties of nickel(II) oxide thin films prepared by aerosol-assisted chemical vapor deposition. ACS Appl Mater Interfaces 5:5675–5682. https://doi.org/10.1021/am401025v
Hammad AH, Abdel-wahab MS, Vattamkandathil S (2019) Growth and correlation of the physical and structural properties of hexagonal nanocrystalline nickel oxide thin films with film thickness. Coatings 9(10):615. https://doi.org/10.3390/coatings9100615
Kaviyarasu K, Manikandan E, Kennedy J, Jayachandran M (2016) Synthesis and characterization studies of NiO nanorods for enhancing solar cell efficiency using photon upconversion materials. Ceram Int 42(7):8385–8394. https://doi.org/10.1016/j.ceramint.2016.02.054
Hassan AJ (2014) Study of optical and electrical properties of nickel oxide (NiO) thin films deposited by using a spray pyrolysis technique. J Modern Phys 05(18):2184–2191. https://doi.org/10.4236/jmp.2014.518212
Granqvist CG, Niklasson GA (2009) Electrochromism in nickel oxide and tungsten oxide thin films: ion intercalation from different electrolytes. Sol Energy Mater Sol Cells 93:2050–2055. https://doi.org/10.1016/j.solmat.2009.05.009
Usha KS, Sivakumar R, Sanjeeviraja C, Sathe V, Ganesan V, Wang TY (2016) Improved electrochromic performance of a radio frequency magnetron sputtered NiO thin film with high optical switching speed. RSC Adv 6:79668–79680. https://doi.org/10.1039/c5ra27099e
Granqvist CG (2002) Handbook of inorganic electrochromic materials. Elsevier Department of Technology School of Engineering University of Uppsala, Sweden
Korošec RC, Felicijan M, Žener B, Pompe M, Dražić G, Gomilšek JP, Pihlar B, Bukovec P (2017) The role of thermal analysis in optimization of electrochromic effect of nickel oxide thin films, prepared by the sol-gel method: part III. Thermochim Acta 655:344–350. https://doi.org/10.1016/j.tca.2017.07.010
Boschloo G, Hagfeldt A (2001) Spectroelectrochemistry of Nanostructured NiO. J Phys Chem B 105:3039–3044. https://doi.org/10.1021/jp003499s
Kondalkar VV, Patil PB, Mane RM, Patil PS, Choudhury S, Bhosal PN (2016) Electrochromic performance of nickel oxide thin film: synthesis via electrodeposition technique. Macromol Symp 361:47–50. https://doi.org/10.1002/masy.201400253
Browne MP, Nolan H, Berner NC, Duesberg G, Colavita P, Lyons M (2016) Electrochromic nickel oxide films for smart window applications. Int J Electrochem Sci 11:6636–6647. https://doi.org/10.20964/2016.08.38
Kamal H, Elmaghraby EK, Ali SA, Abdel-hady K (2005) The electrochromic behavior of nickel oxide films sprayed at different preparative conditions. Thin Solid Films 483:330–339. https://doi.org/10.1016/j.tsf.2004.12.022
Cesiulis H, Tsyntsaru N, Ramanavicius A, Ragoisha G (2016) The study of thin films by electrochemical impedance spectroscopy. Nanotechnol Nanosci. https://doi.org/10.1007/978-3-319-30198-3
Gibson EA, Awais M, Dini D, Dowling DP, Pryce MT, Vos JG, Hagfeldt A (2013) Dye sensitized solar cells with nickel oxide photocathodes prepared via scalable microwave sintering. Phys Chem Chem Phys 15:2411–2420. https://doi.org/10.1039/c2cp43592f
Chou CS, Hsiung CM, Wang CP, Yang RY, Guo MG (2010) Preparation of a counter electrode with p -type nio and its applications in dye-sensitized solar cell. Int J Photoenergy. https://doi.org/10.1155/2010/902385
Gibson EA, Smeigh AL, Pleux LL, Fortage J, Boschloo G, Blart E, Pellegrin Y, Odobel F, Hagfeldt A, Hammarström L (2009) A p-type NiO-based dye-sensitized solar cell with an open-circuit. Angew Chem Int Ed Engl 48(24):4402–4405. https://doi.org/10.1002/anie.200900423
He J, Lindström H, Hagfeldt A, Lindquist SE (1999) Dye-sensitized nanostructured p-type nickel oxide film as a photocathode for a solar cell. J Phys Chem B 103:8940–8943. https://doi.org/10.1021/jp991681r
Bandara J, Pradeep UW, Bandara RGSJ (2004) The role of n–p junction electrodes in minimizing the charge recombination and enhancement of photocurrent and photovoltage in dye sensitized solar cells. J Photochem Photobiol 170(3):273–278. https://doi.org/10.1016/j.jphotochem.2004.08.023
D’Amario L, Boschloo G, Hagfeldt A, Hammarström L (2014) Tuning of conductivity and density of states of NiO mesoporous films used in p-type DSSCs. J Phys Chem C 118(34):19556–19564. https://doi.org/10.1021/jp504551v
Acknowledgements
The authors acknowledge Indira Gandhi Delhi Technical University for Women (IGDTUW), New Delhi, India, and Netaji Subhas University of Technology (NSUT), New Delhi, India for their immense support and infrastructure facilities in research work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Material preparation, data collection and analysis were performed by RG, RJ, CR. The first draft of the manuscript was written by RG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goel, R., Jha, R. & Ravikant, C. Solution-processed spin coated multilayer structured nickel oxide thin films for anodic electrochromism. J Appl Electrochem 53, 713–735 (2023). https://doi.org/10.1007/s10800-022-01807-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01807-6