Abstract
The concentration at which the camel-bell shape transition occurs in the capacitance curve of electric double layer capacitors is predicted via a new regularity stating that various plots of the integral capacitance, related to different surface charge densities, versus concentration (i.e., \({C}_{s}-C\) curves) intersect at a specific concentration. Interestingly, the concentration at the intersection point is found to correspond to that of the concentration of camel-bell shape transition. The existence of such intersection reveals that integral capacitance increases (decreases) with surface charge density for concentrations smaller (or, larger) than that corresponding to the intersection point. Using the modified fundamental measure theory within the framework of the restricted primitive model, this study explores the effects of wall curvature and its convexity or concavity on this regularity and the characteristics of the intersection point. Results show that the intersection point for a convex wall occurs at concentrations larger than those observed for concave walls. The new regularity described is found valid for the capacitance of electric double layers at the interface with walls of different curvatures, including the convex wall of a charged spherical colloid particle and concave wall of a charged spherical cavity as well as planar and cylindrical pores. Furthermore, it is shown that the regularity in question can be used to predict the concentration at which the observed minimum in the asymmetric capacitance curve disappears in the case of asymmetric electrolytes.
Graphic abstract



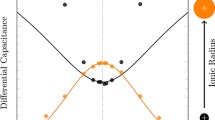
adapted from Ref. [33] and the solid lines represent DFT calculations






Similar content being viewed by others
References
Wang F, Wu X, Yuan X, Liu Z, Zhang Y, Fu L, Zhu Y, Zhou Q, Wu Y, Huang W (2017) Latest advances in supercapacitors: from new electrode materials to novel device designs. Chem Soc Rev 46(22):6816–6854
Zhan C, Lian C, Zhang Y, Thompson MW, Xie Y, Wu J, Kent PR, Cummings PT, De J, Wesolowski DJ (2017) Computational insights into materials and interfaces for capacitive energy storage. Adv Sci 4(7):1700059
Wang X, Mehandzhiyski AY, Arstad B, Van Aken KL, Mathis TS, Gallegos A, Tian Z, Ren D, Sheridan E, Grimes BA (2017) Selective charging behavior in an ionic mixture electrolyte-supercapacitor system for higher energy and power. J Am Chem Soc 139(51):18681–18687
Kong X, Wu J, Henderson D (2015) Density functional theory study of the capacitance of single file ions in a narrow cylinder. J Colloid Interface Sci 449:130–135
Henderson D, Lamperski S (2011) Simple description of the capacitance of the double layer of a high concentration electrolyte. J Chem Eng Data 56(4):1204–1208
Lamperski S, Henderson D (2011) Simulation study of capacitance of the electrical double layer of an electrolyte near a highly charged electrode. Mol Simul 37(4):264–268
Henderson D, Lamperski S, Jin Z, Wu J (2011) Density functional study of the electric double layer formed by a high density electrolyte. J Phys Chem B 115(44):12911–12914
Jiang D-e, Meng D, Wu J (2011) Density functional theory for differential capacitance of planar electric double layers in ionic liquids. Chem Phys Lett 504(4–6):153–158
Jiang De, Wu J (2013) Microscopic insights into the electrochemical behavior of nonaqueous electrolytes in electric double-layer capacitors. J Phys Chem Lett 4(8):1260–1267
Neal JN, Wesolowski DJ, Henderson D, Wu J (2018) Electric double layer capacitance for ionic liquids in nanoporous electrodes: Effects of pore size and ion composition. J Mol Liq 270:145–150
Lian C, Jiang De, Liu H, Wu J (2016) A generic model for electric double layers in porous electrodes. J Phys Chem C 120(16):8704–8710
Feng G, Jiang De, Cummings PT (2012) Curvature effect on the capacitance of electric double layers at ionic liquid/onion-like carbon interfaces. J Chem Theory Comput 8(3):1058–1063
Keshavarzi E, Abareghi M, Helmi A (2019) Curvature dependence of the camel-bell curve transition on the capacitance curve of spherical electric double-layer in porous electrodes: density functional theory. Electrochim Acta 313:303–310
Ma K, Woodward CE, Forsman J (2014) Classical density functional study on interfacial structure and differential capacitance of ionic liquids near charged surfaces. J Phys Chem C 118(29):15825–15834
Kornyshev AA (2007) Double-layer in ionic liquids: paradigm change? J Phys Chem B 111(20):5545–5557
Goodwin ZA, Feng G, Kornyshev AA (2017) Mean-field theory of electrical double layer in ionic liquids with account of short-range correlations. Electrochim Acta 225:190–197
Keshavarzi E, Abareghi M (2020) The effect of electro-neutrality violation inside a charged spherical cavity on the capacitance curve shape in DFT approach and interpretation of mean electrostatic potential. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.114271
Fedorov MV, Georgi N, Kornyshev AA (2010) Double layer in ionic liquids: the nature of the camel shape of capacitance. Electrochem Commun 12(2):296–299
Islam MM, Alam MT, Ohsaka T (2008) Electrical double-layer structure in ionic liquids: a corroboration of the theoretical model by experimental results. J Phys Chem C 112(42):16568–16574
Lamperski S, Outhwaite CW, Bhuiyan LB (2009) The electric double-layer differential capacitance at and near zero surface charge for a restricted primitive model electrolyte. J Phys Chem B 113(26):8925–8929
Pizio O, Sokołowski S, Sokołowska Z (2012) Electric double layer capacitance of restricted primitive model for an ionic fluid in slit-like nanopores: a density functional approach. J Chem Phys 137(23):234705
Shen G, Sun Y, Wang Y, Lu X, Ji X (2020) Interfacial structure and differential capacitance of ionic liquid/graphite interface: a perturbed-chain SAFT density functional theory study. J Mol Liq 310:113199. https://doi.org/10.1016/j.molliq.2020.113199
Henderson D, Silvestre-Alcantara W, Kaja M, Lamperski S, Wu J, Bhuiyan LB (2017) Structure and capacitance of an electric double layer of an asymmetric valency dimer electrolyte: a comparison of the density functional theory with Monte Carlo simulations. J Mol Liq 228:236–242
Henderson D (2012) Oscillations in the capacitance of a nanopore containing an electrolyte due to pore width and nonzero size ions. J Colloid Interface Sci 374(1):345–347
Bossa GV, Caetano DL, de Carvalho SJ, Bohinc K, May S (2018) Modeling the camel-to-bell shape transition of the differential capacitance using mean-field theory and Monte Carlo simulations. The Eur Phys J E 41(9):1–9
Trulsson M, Algotsson J, Forsman J, Woodward CE (2010) Differential capacitance of room temperature ionic liquids: the role of dispersion forces. J Phys Chem Lett 1(8):1191–1195
Pizio O, Sokołowski S (2013) Restricted primitive model for electrolyte solutions in slit-like pores with grafted chains: microscopic structure, thermodynamics of adsorption, and electric properties from a density functional approach. J Chem Phys 138(20):204715
Faramarzi E, Maghari A (2017) The effect of dispersion interactions on the structure and performance of electrical double layer of ionic liquids. J Mol Liq 246:325–331
Zhou S (2018) Capacitance of electrical double layer formed inside a single infinitely long cylindrical pore. J Stat Mech 2018(10):103203
Yu Y-X, Wu J, Gao GH (2004) Density-functional theory of spherical electric double layers and ζ potentials of colloidal particles in restricted-primitive-model electrolyte solutions. J Chem Phys 120(15):7223–7233
Mansoori G, Carnahan NF, Starling K, Leland T Jr (1971) Equilibrium thermodynamic properties of the mixture of hard spheres. J Chem Phys 54(4):1523–1525
Rosenfeld Y (1989) Free-energy model for the inhomogeneous hard-sphere fluid mixture and density-functional theory of freezing. Phys Rev Lett 63(9):980
Degrève L, Lozada-Cassou M, Sánchez E, González-Tovar E (1993) Monte Carlo simulation for a symmetrical electrolyte next to a charged spherical colloid particle. J Chem Phys 98(11):8905–8909
Acknowledgements
The authors would like to acknowledge Isfahan University of Technology. Dr. Ezzatollah Roustazadeh from The English Language Center of Isfahan University of Technology also deserves our gratitude for editing the final English manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keshavarzi, E., Rabiei-Jildani, S. & Abareghi, M. A new regularity used to predict the camel-bell shape transition in the capacitance curve of electric double layer capacitors. J Appl Electrochem 51, 1229–1240 (2021). https://doi.org/10.1007/s10800-021-01571-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-021-01571-z