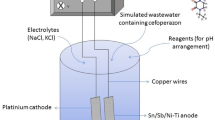
Abstract
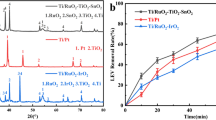
Ceftazidime was selected as target pollutant for electrochemical degradation on two most typical dimensional stable anodes Ti/PbO2–Ce and Ti/SnO2–Sb due to its broad persistence in environment. Degradation experiments were conducted through spiking 5 mg L−1 ceftazidime in real secondary effluents and evaluating the residual antibiotics or COD after 1 h and 4 h electrolysis, respectively. The morphology, composition and electrochemical characteristics of the anodes were compared and it was demonstrated that Ti/SnO2–Sb performed the highest degradation rate because of its superior hydroxyl (·OH) production capability and Ti/PbO2–Ce was regarded as the most stable anode proved by stability tests in 0.5 M aqueous H2SO4 with an anodic current density of 1.0 A. As high as 99.37% of ceftazidime degradation and 95.52% COD removal was achieved on Ti/SnO2–Sb anode under a current of 4 mA cm−1, compared with 75.15% ceftazidime degradation and 83.54% COD removal on Ti/PbO2–Ce. The effects of NaCl and humic acid (HA) in electrolyte were mainly investigated because their existence could interfere the electrochemical degradation process. The experiment results showed NaCl concentration can significantly enhance the oxidation rate with 99% removal within 5 min of treatment on Ti/SnO2–Sb anode, while humic acid concentration is a parameter that inhibited electrochemical catalyzing. Therefore, the degradation efficiency of spiked ceftazidime in real wastewater was evaluated. The last lead to the identification of intermediates during 150 min degradation process and a pathway scheme of ceftazidime was proposed.
Graphic abstract









Similar content being viewed by others
References
Kümmerer K (2009) Antibiotics in the aquatic environment—a review—part I. Chemosphere 75:417–434
Wang W, Wang H, Zhang W et al (2017) Occurrence, distribution, and risk assessment of antibiotics in the Songhua River in China. Environ Sci Pollut Res Int 24:1–11
Mirzaei R, Yunesian M, Nasseri S et al (2018) Occurrence and fate of most prescribed antibiotics in different water environments of Tehran. Iran Sci Total Environ 619–620:446–459
Yu X, Tang X, Zuo J et al (2016) Distribution and persistence of cephalosporins in cephalosporin producing wastewater using SPE and UPLC–MS/MS method. Sci Total Environ 569–570:23–30
Blair B, Nikolaus A, Hedman C et al (2015) Evaluating the degradation, sorption, and negative mass balances of pharmaceuticals and personal care products during wastewater treatment. Chemosphere 134:395–401
Dai G, Huang J, Chen W et al (2014) Major pharmaceuticals and personal care products (PPCPs) in wastewater treatment plant and receiving water in Beijing, China, and associated ecological risks. Bull Environ Contam Toxicol 92:655–661
Wang J, Wang S (2016) Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J Environ Manage 182:620–640
Capodaglio AG, Bojanowska-Czajka A, Trojanowicz M (2018) Comparison of different advanced degradation processes for the removal of the pharmaceutical compounds diclofenac and carbamazepine from liquid solutions. Environ Sci Pollut Res 25:27704
Oturan MA, Aaron J-J (2014) advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit Rev Environ Sci Technol 44:2577–2641
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36:1–84
Sirés I, Brillas E (2012) Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: a review. Environ Int 40:212–229
Feng L, van Hullebusch ED, Rodrigo MA et al (2013) Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem Eng J 228:944–964
Martínez-Huitle CA, Panizza M (2018) Electrochemical oxidation of organic pollutants for wastewater treatment. Curr Opin Electrochem 11:62–71
Labiadh L, Barbucci A, Carpanese MP et al (2017) Direct and indirect electrochemical oxidation of Indigo Carmine using PbO2 and TiRuSnO2. J Solid State Electrochem 21:2167–2175
Ganiyu SO, Oturan N, Raffy S et al (2019) Efficiency of plasma elaborated sub-stoichiometric titanium oxide (Ti4O7) ceramic electrode for advanced electrochemical degradation of paracetamol in different electrolyte media. Sep Purif Technol 208:142–152
De Moura DC, De Araújo CKC, Zanta CLPS et al (2014) Active chlorine species electrogenerated on Ti/Ru0.3Ti0.7O2 surface: electrochemical behavior, concentration determination and their application. J Electroanal Chem 731:145–152
Ganiyu SO, Oturan N, Raffy S et al (2016) Sub-stoichiometric titanium oxide (Ti4O7) as a suitable ceramic anode for electrooxidation of organic pollutants: a case study of kinetics, mineralization and toxicity assessment of amoxicillin. Water Res 106:171–182
Liu H, Liu Y, Zhang C, Shen R (2008) Electrocatalytic oxidation of nitrophenols in aqueous solution using modified PbO2 electrodes. J Appl Electrochem 38:101–108
Ciriaco L, Santos D, Pacheco MJ, Lopes A (2011) Anodic oxidation of organic pollutants on a Ti/SnO2-Sb2O4 anode. J Appl Electrochem 41:577–587
Santos D, Pacheco MJ, Gomes A et al (2013) Preparation of Ti/Pt/SnO2-Sb2O4 electrodes for anodic oxidation of pharmaceutical drugs. J Appl Electrochem 43:407–416
Duan XY, Li JR, Chang LM, Yang CW (2016) A comparison of electrochemical oxidation performance of PbO2 and SnO2 electrodes. J Water Reuse Desalin 6:392–398
Shmychkova O, Luk’yanenko T, Yakubenko A et al (2015) Electrooxidation of some phenolic compounds at Bi-doped PbO2. Appl Catal B-Environ 162:346–351
Shmychkova O, Luk’yanenko T, Amadelli R, Velichenko A (2016) Electrodeposition of Ni2+-doped PbO2 and physicochemical properties of the coating. J Electroanal Chem 774:88–94
Luk’yanenko T, Shmychkova O, Velichenko A (2020) PbO2-surfactant composites: electrosynthesis and catalytic activity. J Solid State Electrochem 24:1045–1056
Shmychkova O, Lukyanenko T, Amadelli R, Velichenko A (2014) Physico-chemical properties of PbO2-anodes doped with Sn4+ and complex ions. J Electroanal Chem 717:196–201
Sirés I, Brillas E, Oturan MA et al (2014) Electrochemical advanced oxidation processes: today and tomorrow. A review. Environ Sci Pollut Res 21:8336–8367
Moreira FC, Boaventura RAR, Brillas E, Vilar VJP (2017) Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl Catal B Environ 202:217–261
Komtchou S, Dirany A, Drogui P et al (2017) Removal of atrazine and its by-products from water using electrochemical advanced oxidation processes. Water Res 125:91–103
Zhi D, Qin J, Zhou H et al (2017) Removal of tetracycline by electrochemical oxidation using a Ti/SnO2-Sb anode: characterization, kinetics, and degradation pathway. J Appl Electrochem 47:1313–1322
Hu X, Yu Y, Sun Z (2016) Preparation and characterization of cerium-doped multiwalled carbon nanotubes electrode for the electrochemical degradation of low-concentration ceftazidime in aqueous solutions. Electrochim Acta 199:80–91
Duan X, Li J, Liu W et al (2016) Fabrication and characterization of a novel PbO2 electrode with a CNT interlayer. RSC Adv 6:28927–28936
Li L, Huang Z, Fan X et al (2017) Preparation and characterization of a Pd modified Ti/SnO2-Sb anode and its electrochemical degradation of Ni-EDTA. Electrochim Acta 231:354–362
Lei X, Li L, Chen Y, Hu Y (2018) Effect of calcination temperature on the properties of Ti/SnO2-Sb anode and its performance in Ni-EDTA electrochemical degradation. Environ Sci Pollut Res 25:11683–11693
Radjenovic J, Sedlak DL (2015) Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ Sci Technol 49:11292–11302
Kwon BG, Ryu S, Yoon J (2009) Determination of hydroxyl radical rate constants in a continuous flow system using competition kinetics. J Ind Eng Chem 15:809–812
Sopaj F, Oturan N, Pinson J et al (2016) Effect of the anode materials on the efficiency of the electro-Fenton process for the mineralization of the antibiotic sulfamethazine. Appl Catal B Environ 199:331–341
Wu W, Huang ZH, Lim TT (2014) Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water. Appl Catal A Gen 480:58–78
Yang K, Liu Y, Qiao J (2017) Electrodeposition preparation of Ce-doped Ti/SnO2-Sb electrodes by using selected addition agents for efficient electrocatalytic oxidation of methylene blue in water. Sep Purif Technol 189:459–466
Yang B, Jiang C, Yu G et al (2015) Highly efficient electrochemical degradation of perfluorooctanoic acid (PFOA) by F-doped Ti/SnO2 electrode. J Hazard Mater 299:417–424
Wu W, Huang ZH, Hu ZT et al (2017) High performance duplex-structured SnO2-Sb-CNT composite anode for bisphenol A removal. Sep Purif Technol 179:25–35
Duan X, Zhao C, Liu W et al (2017) Fabrication of a novel PbO2 electrode with a graphene nanosheet interlayer for electrochemical oxidation of 2-chlorophenol. Electrochim Acta 240:424–436
Dobrosz-Gomez I, Gomez MAG, Gaviria GH, GilPavas E (2020) Mineralization of cyanide originating from gold leaching effluent using electro-oxidation: multi-objective optimization and kinetic study. J Appl Electrochem 50:217–230
García-Espinoza JD, Mijaylova-Nacheva P, Avilés-Flores M (2018) Electrochemical carbamazepine degradation: effect of the generated active chlorine, transformation pathways and toxicity. Chemosphere 192:142–151
Mogyorody F (2006) Influence of chlorine-water equilibria on the electrochemical destruction of thiocarbamate herbicides in NaCl solutions. J Appl Electrochem 36:765–771
Deborde M, von Gunten U (2008) Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanisms: a critical review. Water Res 42:13–51
He X, Mezyk SP, Michael I et al (2014) Degradation kinetics and mechanism of β-lactam antibiotics by the activation of H2O2 and Na2S2O8 under UV-254nm irradiation. J Hazard Mater 279:375–383
Dries J, De SA, Goethals L et al (1998) High rate biological treatment of sulfate-rich wastewater in an acetate-fed EGSB reactor. Biodegradation 9:103–111
Acknowledgements
This research was financially supported by the National Water Pollution Control and Management Technology Major Projects (2018ZX07601003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, P., Jia, X., Lin, J. et al. Electro-oxidation of ceftazidime in real municipal wastewater using PbO2–Ce and SnO2–Sb electrodes: influence of electrolyte and degradation pathway. J Appl Electrochem 51, 183–195 (2021). https://doi.org/10.1007/s10800-020-01482-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-020-01482-5