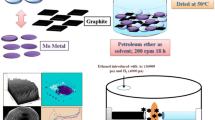
Abstract
An interwoven hollow fiber structured mesoporous NiO catalyst doped with C, S was fabricated by the vacuum impregnation and thermal decomposition methods using eggshell membrane as template. The structure of the catalyst was characterized by X-ray diffraction, thermo-gravimetric analysis, scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, and specific surface area test. Under a high vacuum condition, the synthesized NiO material has a better interwoven fiber and mesoporous structure, which effectively increases the specific surface area of the material. C and S were doped into NiO via eggshell membrane pyrolysis, which then formed C–OH and SO42− species on the surface of the material. The electrocatalytic performance of the catalyst for the oxidation of methanol in alkaline solution was studied by cyclic voltammetry and chronoamperometry. The C, S doped mesoporous NiO material exhibits much higher catalytic efficiency and anti-poisoning ability than that of NiO nanoparticles due to the synergistic catalysis of NiO and doping C–OH and SO42− species.
Graphic abstract
















Similar content being viewed by others
References
Abrego-Martínez J, Wang Y, Moreno-Zuria A, Wei Q, Cuevas-Muniz F, Arriaga L, Sun S, Mohamedi M (2019) Nanostructured Mn2O3/Pt/CNTs selective electrode for oxygen reduction reaction and methanol tolerance in mixed-reactant membraneless micro-DMFC. Electrochim Acta 297:230–239
Ong BC, Kamarudin SK, Basri S (2017) Direct liquid fuel cells: a review. Int J Hydrogen Energy 42(15):10142–10157
Antolini E (2016) Structural parameters of supported fuel cell catalysts: the effect of particle size, inter-particle distance and metal loading on catalytic activity and fuel cell performance. Appl. Catal. B 181:298–313
Neelakandan S, Muthumeenal A, Rana D, Kaleekkal NJ, Nagendran A (2019) Sulfonated poly (phenylene ether ether sulfone) membrane tailored with layer-by-layer self-assembly of poly (diallyldimethylammonium chloride) and phosphotungstic acid for DMFC applications. J Appl Polym Sci 136(16):47344–47351
Kamarudin SK, Achmad F, Daud WRW (2009) Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int J Hydrogen Energy 34(16):6902–6916
Carrette L, Friedrich KA, Stimming U (2015) Fuel cells—fundamentals and applications. Fuel Cells 1(1):5–39
Zhang K, Chen X, Wang L, Zhang D, Xue Z, Zhou X, Lu X (2018) Pt-Pd nanoparticles supported on sulfonated nitrogen sulfur co-doped graphene for methanol electro-oxidation. Int J Hydrogen Energy 43(33):15931–15940
Li P, Gu Y, Yu Z, Gao P, An Y, Li J (2019) TiO2-SnO2/SO42− mesoporous solid superacid decorated nickel-based material as efficient electrocatalysts for methanol oxidation reaction. Electrochim Acta 297:864–871
Yang Z, Nakashima N (2015) A simple preparation of very high methanol tolerant cathode electrocatalyst for direct methanol fuel cell based on polymer-coated carbon nanotube/platinum. Sci Rep 5:12236–12245
Shukla AK, Raman RK, Scott K (2010) Advances in mixed-reactant fuel cells. Fuel Cells 5(4):436–447
Hui L, Ling L, Yang Z, Yang Y (2010) Hydrothermal synthesis of ultralong single-crystalline α-Ni(OH)2 nanobelts and corresponding porous NiO nanobelts. Cryst Res Technol 45(6):661–666
Lihong D, Ying C, Wendong S (2010) Controllable synthesis of nickel hydroxide and porous nickel oxide nanostructures with different morphologies. Chem Eur J 14(16):5064–5072
Zhuo L, Ge J, Cao L, Bo T (2009) Solvothermal synthesis of CoO, Co3O4, Ni(OH)2 and Mg(OH)2 nanotubes. Cryst Growth Des 9(1):1–6
Rath MK, Choi BH, Ji MJ, Lee KT (2014) Eggshell-membrane-templated synthesis of hierarchically-ordered NiO–Ce0.8Gd0.2O1.9 composite powders and their electrochemical performances as SOFC anodes. Ceram. Int. 40(2):3295–3304
Dong Q, Su H, Zhang D, Guo Q, Kiessling F (2008) Fabrication of hierarchical ZnO films with interwoven porous conformations by a bioinspired templating technique. Chem Eng J 137(2):428–435
He H, Yang P (2018) CeO2/NiO nanostructures created using eggshell membrane towards enhanced catalytic activity. J Nanosci Nanotechnol 18(1):340–346
Ma JH, Wang L, Mu X, Li L (2015) Nitrogen-doped graphene supported Pt nanoparticles with enhanced performance for methanol oxidation. Int J Hydrogen Energy 40(6):2641–2647
Sun Y, Du C, An M, Lei D, Qiang T, Liu C, Gao Y, Yin G (2015) Boron-doped graphene as promising support for platinum catalyst with superior activity towards the methanol electrooxidation reaction. J Power Sources 300(10):245–253
Kanninen P, Luong ND, Flórez-Montaño J, Jiang H, Pastor E, Seppälä J, Kallio T (2017) Highly active platinum nanoparticles supported by nitrogen/sulfur functionalized graphene composite for ethanol electro-oxidation. Electrochim Acta 242:315–326
Zhao SL, Yin HJ, Lei D, Yin GP, Tang ZY, Liu SQ (2014) Three dimensional N-doped graphene/PtRu nanoparticle hybrids as high performance anode for direct methanol fuel cells. J Mater Chem A 2(11):3719–3724
Zhang W, Huang H, Li F, Deng K, Wang X (2014) Palladium nanoparticles supported on graphitic carbon nitride-modified reduced graphene oxide as highly efficient catalysts for formic acid and methanol electrooxidation. J Mater Chem A 2(44):19084–19094
An M, Du C, Du L, Wang Y, Wang Y, Sun Y, Yin G, Gao Y (2019) Enhanced methanol oxidation in acid media on Pt/S, P Co-doped graphene with 3D porous network structure engineering. ChemElectroChem 6(4):1157–1165
Liu X, Yin C, Yang J, Liang M, Wei J, Zhang Z, Wang H, Wang Q (2016) Controllable preparation of an eggshell membrane supported hydrogel electrolyte with thickness-dependent electrochemical performance. J Mater Chem A 4(46):17933–17938
Pant B, Park M, Kim HY, Park SJ (2017) CdS-TiO2 NPs decorated carbonized eggshell membrane for effective removal of organic pollutants: a novel strategy to use a waste material for environmental remediation. J Alloys Compd 699(2017):73–78
Zhong SL, Zhuang J, Yang DP, Tang D (2017) Eggshell membrane-templated synthesis of 3D hierarchical porous Au networks for electrochemical nonenzymatic glucose sensor. Biosens Bioelectron 96:26–32
Fan S, Zhao M, Ding L, Liang J, Jing C, Li Y, Chen S (2016) Synthesis of 3D hierarchical porous Co3O4 film by eggshell membrane for non-enzymatic glucose detection. J Electroanal Chem 775(8):52–57
Meng X, Deng D (2019) Bio-inspired synthesis of 3-D network of NiO-Ni nanowires on carbonized eggshell membrane for lithium-ion batteries. Chem Eng Sci 194:134–141
Wang Z, Shao X, Hu X, Parkinson G, Xie K, Dong D, Li CZ (2014) Hierarchically structured NiO/CeO2 nanocatalysts templated by eggshell membranes for methane steam reforming. Catal Today 228(228):199–205
Zhang S, Shao Y, Liao HG, Liu J, Aksay IA, Yin G, Lin Y (2011) Graphene decorated with PtAu alloy nanoparticles: facile synthesis and promising application for formic acid oxidation. Chem Mater 23(5):1079–1081
He C, Kunz HR, Fenton JM (1997) Evaluation of platinum-based catalysts for methanol electro-oxidation in phosphoric acid electrolyte. J Electrochem Soc 144(3):970
Yu H, Tang Q, Wu J, Lin Y, Fan L, Huang M, Lin J, Yan L, Yu F (2012) Using eggshell membrane as a separator in supercapacitor. J Power Sources 206(206):463–468
Camaratta R, Lima ANC, Reyes MD, Hernández-Fenollosa MA, Messana JO, Bergmann CP (2013) Microstructural evolution and optical properties of TiO2 synthesized by eggshell membrane templating for DSSCs application. Mater Res Bull 48(4):1569–1574
Li Y, Ji Z, Fan Y, Yong Y, Tang B (2017) Preparation of environment-friendly 3D eggshell membrane-supported anatase TiO2 as a reusable photocatalyst for degradation of organic dyes. Chem Phys Lett 689:142–147
Yang D, Qi L, Ma J (2003) Hierarchically ordered networks comprising crystalline ZrO2 tubes through sol–gel mineralization of eggshell membranes. J Mater Chem 13(5):1119–1123
Gong C, Zhou Z, Zhou H, Liu R (2019) Vacuum-assisted synthesis of tiny Au nanoparticles entrapped into mesoporous carbon matrix with superior catalytic activity for 4-nitrophenol reduction. Adv Powder Technol 30(3):649–655
Sun S, Wang L, Wang C, Zhang Y (2018) Vacuum-assisted synthesis of spherical activated carbon using coal tar pitch with low softening point by copolymerization. Vacuum 158:215–217
Fu Q, Yang P, Wang J, Wang H, Yang L, Zhao X (2018) In situ synthesis of Ni nanofibers via vacuum thermal reduction and their efficient catalytic properties for hydrogen generation. J Mater Chem A 6(24):11370–11376
Sun J, Zhu M, Fan M, He J, Han C, Yang G, Shan Y (2019) Mo2C-Ni modified carbon microfibers as an effective electrocatalyst for hydrogen evolution reaction in acidic solution. J Colloid Interface Sci 543:300–306
Liu H, Zeng S, He P, Dong F, He M, Zhang Y, Wang S, Li C, Liu M, Jia L (2019) Samarium oxide modified Ni-Co nanosheets based three-dimensional honeycomb film on nickel foam: a highly efficient electrocatalyst for hydrogen evolution reaction. Electrochim Acta 299:405–414
Xu W, Chao C, Du Y, Liu XZ, Li X, Xie X (2018) Facile synthesis of NiAl-LDHs with tunable establishment of acid-base activity sites. Mater Chem Phys 211:72–78
Zhang X, Zhang B, Liu S, Kang H, Kong W, Zhang S, Yan S, Yang B (2018) RGO modified Ni doped FeOOH for enhanced electrochemical and photoelectrochemical water oxidation. Appl Surf Sci 436:974–980
Lu L, Xin Z, Wang X, Wang S, Zhu H, Li T, Gu Y, Yan S, Zou Z (2019) KOH-modified Ni/LaTiO2N Schottky junction efficiently reducing CO2 to CH4 under visible light irradiation. Appl Catal B 244:786–794
Wei L, Guo RT, Wang SX, Pan WG, Chen QL, Li MY, Peng S, Liu SM (2016) The enhanced Zn resistance of Mn/TiO2 catalyst for NH3-SCR reaction by the modification with Nb. Fuel Process Technol 154:235–242
Hino M, Kurashige M, Matsuhashi H, Arata K (2006) The surface structure of sulfated zirconia: studies of XPS and thermal analysis. Thermochim Acta 441(1):35–41
Min Z, Hui Q, Qiu H, Tong Z, Zhao X, Yue H, Gang C, Wang C, Wei Y, Dong Z (2018) Reduced graphene oxide wrapped alluaudite Na2+2xFe2-x(SO) with high rate sodium ion storage properties. J Alloys Compd 752:267–273
Yan GX, Wang A, Wachs IE, Baltrusaitis J (2019) Critical review on the active site structure of sulfated zirconia catalysts and prospects in fuel production. Appl Catal A 572:210–225
Iwasita T (2002) Electrocatalysis of methanol oxidation. Electrochim Acta 47(22):3663–3674
Abdel Rahim MA, Abdel Hameed RM, Khalil MW (2004) Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J Power Sources 134(2):160–169
Hu X, Chen Y, Huang B, Liu Y, Huang H, Xie Z (2019) Pd supported N/S codoped graphene-like carbons boost quinoline hydrogenation activity. ACS Sustain Chem Eng 7(13):11369–11376
Ojani R, Abkar Z, Hasheminejad E, Raoof JB (2014) Rapid fabrication of Cu/Pd nano/micro-particles porous-structured catalyst using hydrogen bubbles dynamic template and their enhanced catalytic performance for formic acid electrooxidation. Int J Hydrogen Energy 39(15):7788–7797
Malode SJ, Shetti NP, Nandibewoor ST (2012) Voltammetric behavior of theophylline and its determination at multi-wall carbon nanotube paste electrode. Colloids Surf B 97:1–6
Hegde RN, Hosamani RR, Nandibewoor ST (2009) Electrochemical oxidation and determination of theophylline at a carbon paste electrode using cetyltrimethyl ammonium bromide as enhancing agent. Anal Lett 42(16):2665–2682
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem Interfacial Electrochem 101(1):19–28
Bard AJ, Faulkner LR (2001) Fundamentals and applications. Electrochem Methods 2(482):580–632
Fleischmann M, Korinek K, Pletcher D (1971) The oxidation of organic compounds at a nickel anode in alkaline solution. J Electroanal Chem Interfacial Electrochem 31(1):39–49
Zhou M, Ouyang R, Li Y, Miao Y (2017) 3D microspheres organized from Ni-Mo-S nanoparticles in situ synthesized on porous Ti for hydrogen evolution electrocatalysis. Electrochim Acta 246:9–16
Zhou M, Liu Y, Fa D, Qian L, Miao Y (2018) Growth of radial microspheres of Ni-Co-O at porous Ti and its phosphorization for high efficient hydrogen evolution. Electrochim Acta 259:329–337
Jeon MK, Won JY, Lee KR, Woo SI (2007) Highly active PtRuFe/C catalyst for methanol electro-oxidation. Electrochem Commun 9(9):2163–2166
Chang J, Feng L, Jiang K, Xue H, Cai WB, Liu C (2016) Pt–CoP/C as an alternative PtRu/C catalyst for direct methanol fuel cells. J Mater Chem A 4(47):18607–18613
Torabi M, Karimi Shervedani R, Amini A (2018) High performance porous graphene nanoribbons electrodes synthesized via hydrogen plasma and modified by Pt-Ru nanoclusters for charge storage and methanol oxidation. Electrochim Acta 290:616–625
Asgari M, Maragheh MG, Davarkhah R, Lohrasbi E (2011) Methanol electrooxidation on the nickel oxide nanoparticles/multi-walled carbon nanotubes modified glassy carbon electrode prepared using pulsed electrodeposition. J Electrochem Soc 158(12):K225–K229
Yaqoob L, Noor T, Iqbal N, Nasir H, Zaman N (2019) Development of nickel-BTC-MOF-derived nanocomposites with rGO towards electrocatalytic oxidation of methanol and its product analysis. Catal 9(10):856–877
Tong Y, Gu C, Zhang J, Huang M, Tang H, Wang X, Tu J (2015) Three-dimensional astrocyte-network Ni–P–O compound with superior electrocatalytic activity and stability for methanol oxidation in alkaline environments. J Mater Chem A 3(8):4669–4678
Barakat NA, Abdelkareem MA, El-Newehy M, Kim HY (2013) Influence of the nanofibrous morphology on the catalytic activity of NiO nanostructures: an effective impact toward methanol electrooxidation. Nanoscale Res Lett 8(1):1–6
Acknowledgements
We acknowledge the National Natural Science Foundation of China (21603143 and 21505092) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, Y., Yu, Z., Wu, S. et al. Eggshell-membrane-templated synthesis of C, S Doped Mesoporous NiO for methanol oxidation in alkaline solution. J Appl Electrochem 50, 821–834 (2020). https://doi.org/10.1007/s10800-020-01438-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-020-01438-9