Abstract
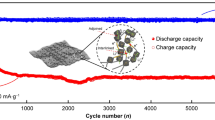
Silicon as a promising candidate for the next-generation high-capacity lithium-ion battery anode is characterized by outstanding capacity, high abundance, low operational voltage, and environmental benignity. However, large volume changes during Si lithiation and de-lithiation can seriously impair its long-term cyclability. Although extensive research efforts have been made to improve the electrochemical performance of Si-based anodes, there is a lack of efficient fabrication methods that are low cost, scalable, and self-assembled. In this report, co-axial fibrous silicon asymmetric membrane has been synthesized using a scalable and straightforward phase inversion method combined with dip coating as inspired by the hollow fiber membrane technology that has been successfully commercialized over the last decades to provide billions of gallons of purified drinking water worldwide. We demonstrate that ~ 90% initial capacity of co-axial fibrous Si asymmetric membrane electrode can be maintained after 300 cycles applying a current density of 400 mA g−1. The diameter of fibers, size of silicon particles, type of polymers, and exterior coating have been identified as critical factors that can influence the electrode stability, initial capacity, and rate performance. Much enhanced electrochemical performance can be harvested from a sample that has thinner fiber diameter, smaller silicon particle, lower silicon content, and porous carbon coating. This efficient and scalable approach to prepare high-capacity silicon-based anode with outstanding cyclability is fully compatible with industrial roll-to-roll processing technology, thus bearing a great potential for its future commercialization.
Graphic abstract








Similar content being viewed by others
Abbreviations
- LIBs:
-
Lithium-ion batteries
- NPs:
-
Nanoparticles
- Si:
-
Silicon
- SEM:
-
Scanning electron microscope
- EDS:
-
Energy-dispersive X-ray spectroscopy
- PXRD:
-
Powder X-ray diffractometer
- TGA:
-
Thermogravimetric analyzer
- BET:
-
Brunauer–Emmett–Teller
- XPS:
-
X-ray photoelectron spectroscopy
- PAN:
-
Polyacrylonitrile
- PS:
-
Polysulfone
- CB:
-
Carbon black
- NMP:
-
N-methyl-2-pyrrolidone
- EIS:
-
Electrochemical impedance spectroscopy
- G:
-
Gauge
- SEI:
-
Solid electrolyte interphase
- rpm:
-
Rotations per minute
References
Chen H, Cong TN, Yang W, Tan C, Li Y, Ding Y (2009) Progress in electrical energy storage system: a critical review. Prog Nat Sci 19(3):291–312. https://doi.org/10.1016/j.pnsc.2008.07.014
Tarascon J-M (2010) Key challenges in future Li-battery research. Philos Trans R Soc A 368(1923):3227–3241. https://doi.org/10.1098/rsta.2010.0112
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652. https://doi.org/10.1038/451652a
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18(5):252–264. https://doi.org/10.1016/j.mattod.2014.10.040
Kim H, Lee E-J, Sun Y-K (2014) Recent advances in the Si-based nanocomposite materials as high capacity anode materials for lithium ion batteries. Mater Today 17(6):285–297. https://doi.org/10.1016/j.mattod.2014.05.003
Su X, Wu Q, Li J, Xiao X, Lott A, Lu W, Sheldon BW, Wu J (2014) Silicon-based nanomaterials for lithium-ion batteries: a review. Adv Energy Mater 4(1):1300882. https://doi.org/10.1002/aenm.201300882
Feng K, Li M, Liu W, Kashkooli AG, Xiao X, Cai M, Chen Z (2018) Silicon-based anodes for lithium-ion batteries: from fundamentals to practical applications. Small 14(8):1702737. https://doi.org/10.1002/smll.201702737
Wu H, Chan G, Choi JW, Ryu I, Yao Y, McDowell MT, Lee SW, Jackson A, Yang Y, Hu L, Cui Y (2012) Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat Nanotechnol 7:310. https://doi.org/10.1038/nnano.2012.35
Wang C, Wu H, Chen Z, McDowell MT, Cui Y, Bao Z (2013) Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat Chem 5:1042. https://doi.org/10.1038/nchem.1802
Magasinski A, Zdyrko B, Kovalenko I, Hertzberg B, Burtovyy R, Huebner CF, Fuller TF, Luzinov I, Yushin G (2010) Toward efficient binders for Li-ion battery Si-based anodes: polyacrylic acid. ACS Appl Mater Interfaces 2(11):3004–3010. https://doi.org/10.1021/am100871y
Kumar SK, Ghosh S, Malladi SK, Nanda J, Martha SK (2018) Nanostructured silicon-carbon 3D electrode architectures for high-performance lithium-ion batteries. ACS Omega 3(8):9598–9606. https://doi.org/10.1021/acsomega.8b00924
Benítez A, Lecce D, Elia GA, Caballero Á, Morales J, Hassoun J (2018) A lithium-ion battery using a 3 D-array nanostructured graphene-sulfur cathode and a silicon oxide-based anode. Chemsuschem 11(9):1512–1520. https://doi.org/10.1002/cssc.201800242
Liu J, Kopold P, van Aken PA, Maier J, Yu Y (2015) Energy storage materials from nature through nanotechnology: a sustainable route from reed plants to a silicon anode for lithium-ion batteries. Angew Chem Int Ed 54(33):9632–9636. https://doi.org/10.1002/anie.201503150
Zhao C, Wada T, De Andrade V, Gürsoy D, Kato H, Y-cK Chen-Wiegart (2018) Imaging of 3D morphological evolution of nanoporous silicon anode in lithium ion battery by X-ray nano-tomography. Nano Energy 52:381–390. https://doi.org/10.1016/j.nanoen.2018.08.009
Magasinski A, Dixon P, Hertzberg B, Kvit A, Ayala J, Yushin G (2010) High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat Mater 9:353. https://doi.org/10.1038/nmat2725
Xia F, Kim SB, Cheng H, Lee JM, Song T, Huang Y, Rogers JA, Paik U, Park WI (2013) Facile synthesis of free-standing silicon membranes with three-dimensional nanoarchitecture for anodes of lithium ion batteries. Nano Lett 13(7):3340–3346. https://doi.org/10.1021/nl401629q
Jin Y, Zhu B, Lu Z, Liu N, Zhu J (2017) Challenges and recent progress in the development of si anodes for lithium-ion battery. Adv Energy Mater 7(23):1700715. https://doi.org/10.1002/aenm.201700715
Petersen RJ (1993) Composite reverse osmosis and nanofiltration membranes. J Membr Sci 83(1):81–150. https://doi.org/10.1016/0376-7388(93)80014-O
Radjenović J, Petrović M, Ventura F, Barceló D (2008) Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res 42(14):3601–3610. https://doi.org/10.1016/j.watres.2008.05.020
Nicolaisen B (2003) Developments in membrane technology for water treatment. Desalination 153(1):355–360. https://doi.org/10.1016/S0011-9164(02)01127-X
Cadotte JE, Petersen RJ, Larson RE, Erickson EE (1980) A new thin-film composite seawater reverse osmosis membrane. Desalination 32:25–31. https://doi.org/10.1016/S0011-9164(00)86003-8
Wu J, Chen H, Padgett C (2016) Silicon asymmetric membranes for efficient lithium storage: a scalable method. Energy Technol 4(4):502–509. https://doi.org/10.1002/ente.201500315
Wu J, Chen H, Byrd I, Lovelace S, Jin C (2016) Fabrication of SnO2 asymmetric membranes for high performance lithium battery anode. ACS Appl Mater Interfaces 8(22):13946–13956. https://doi.org/10.1021/acsami.6b03310
Wu J, Byrd I, Jin C, Li J, Chen H, Camp T, Bujol R, Sharma A, Zhang H (2017) Reinvigorating reverse-osmosis membrane technology to stabilize the V2O5 lithium-ion battery cathode. ChemElectroChem 4(5):1181–1189. https://doi.org/10.1002/celc.201700102
Byrd I, Chen H, Webber T, Li J, Wu J (2015) Self-assembled asymmetric membrane containing micron-size germanium for high capacity lithium ion batteries. RSC Adv 5(113):92878–92884. https://doi.org/10.1039/C5RA19208K
van de Witte P, Dijkstra PJ, van den Berg JWA, Feijen J (1996) Phase separation processes in polymer solutions in relation to membrane formation. J Membr Sci 117(1):1–31. https://doi.org/10.1016/0376-7388(96)00088-9
Wang R, Shi L, Tang CY, Chou S, Qiu C, Fane AG (2010) Characterization of novel forward osmosis hollow fiber membranes. J Membr Sci 355(1):158–167. https://doi.org/10.1016/j.memsci.2010.03.017
Bottino A, Capannelli G, Munari S (1986) Factors affecting the structure and properties of asymmetric polymeric membranes. In: Drioli E, Nakagaki M (eds) Membranes and membrane processes. Springer, Boston, pp 163–178. https://doi.org/10.1007/978-1-4899-2019-5_17
Cocciantelli JM, Ménétrier M, Delmas C, Doumerc JP, Pouchard M, Broussely M, Labat J (1995) On the δ → γ irreversible transformation in Li//V2O5 secondary batteries. Solid State Ionics 78(1–2):143–150. https://doi.org/10.1016/0167-2738(95)00015-X
Du Z, Wood DL, Daniel C, Kalnaus S, Li J (2017) Understanding limiting factors in thick electrode performance as applied to high energy density Li-ion batteries. J Appl Electrochem 47(3):405–415. https://doi.org/10.1007/s10800-017-1047-4
Pinnau I, Koros WJ (1991) Structures and gas separation properties of asymmetric polysulfone membranes made by dry, wet, and dry/wet phase inversion. J Appl Polym Sci 43(8):1491–1502. https://doi.org/10.1002/app.1991.070430811
Kim I-C, Yun H-G, Lee K-H (2002) Preparation of asymmetric polyacrylonitrile membrane with small pore size by phase inversion and post-treatment process. J Membr Sci 199(1):75–84. https://doi.org/10.1016/S0376-7388(01)00680-9
Lee K-W, Seo B-K, Nam S-T, Han M-J (2003) Trade-off between thermodynamic enhancement and kinetic hindrance during phase inversion in the preparation of polysulfone membranes. Desalination 159(3):289–296. https://doi.org/10.1016/S0011-9164(03)90081-6
Barth C, Gonçalves MC, Pires ATN, Roeder J, Wolf BA (2000) Asymmetric polysulfone and polyethersulfone membranes: effects of thermodynamic conditions during formation on their performance. J Membr Sci 169(2):287–299. https://doi.org/10.1016/S0376-7388(99)00344-0
Ferrari AC (2007) Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun 143(1):47–57. https://doi.org/10.1016/j.ssc.2007.03.052
Meier C, Lüttjohann S, Kravets VG, Nienhaus H, Lorke A, Wiggers H (2006) Raman properties of silicon nanoparticles. Phys E 32(1):155–158. https://doi.org/10.1016/j.physe.2005.12.030
Misra S, Liu N, Nelson J, Hong SS, Cui Y, Toney MF (2012) In situ X-ray diffraction studies of (de)lithiation mechanism in silicon nanowire anodes. ACS Nano 6(6):5465–5473. https://doi.org/10.1021/nn301339g
Mondal T, Bhowmick AK, Krishnamoorti R (2012) Chlorophenyl pendant decorated graphene sheet as a potential antimicrobial agent: synthesis and characterization. J Mater Chem 22(42):22481–22487. https://doi.org/10.1039/C2JM33398H
Wang S, Chen Z-H, Ma W-J, Ma Q-S (2006) Influence of heat treatment on physical–chemical properties of PAN-based carbon fiber. Ceram Int 32(3):291–295. https://doi.org/10.1016/j.ceramint.2005.02.014
Huang X (2009) Fabrication and properties of carbon fibers. Materials 2(4):2369
Reynolds WN, Moreton R, Worthington PJ (1980) Some factors affecting the strengths of carbon fibres [and discussion]. Philos Trans R Soc Lond Ser A 294(1411):451–461
He JW, Xu X, Corneille JS, Goodman DW (1992) X-ray photoelectron spectroscopic characterization of ultra-thin silicon oxide films on a Mo(100) surface. Surf Sci 279(1):119–126. https://doi.org/10.1016/0039-6028(92)90748-U
An SJ, Li J, Daniel C, Mohanty D, Nagpure S, Wood DL (2016) The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 105:52–76. https://doi.org/10.1016/j.carbon.2016.04.008
Fitzer E (1989) Pan-based carbon fibers—present state and trend of the technology from the viewpoint of possibilities and limits to influence and to control the fiber properties by the process parameters. Carbon 27(5):621–645. https://doi.org/10.1016/0008-6223(89)90197-8
Ding N, Xu J, Yao YX, Wegner G, Fang X, Chen CH, Lieberwirth I (2009) Determination of the diffusion coefficient of lithium ions in nano-Si. Solid State Ionics 180(2):222–225. https://doi.org/10.1016/j.ssi.2008.12.015
Liu XH, Zhong L, Huang S, Mao SX, Zhu T, Huang JY (2012) Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 6(2):1522–1531. https://doi.org/10.1021/nn204476h
Singh M, Kaiser J, Hahn H (2015) Thick electrodes for high energy lithium ion batteries. J Electrochem Soc 162(7):A1196–A1201. https://doi.org/10.1149/2.0401507jes
Wood M, Li J, Wood DL, Daniel C, Dunlop AR, Polzin BJ, Jansen AN, Krumdick G (2018) Evaluation of thick electrode architectures for high energy density Li-ion batteries. Meeting Abstracts MA2018-01(3):253
Chen LB, Xie JY, Yu HC, Wang TH (2009) An amorphous Si thin film anode with high capacity and long cycling life for lithium ion batteries. J Appl Electrochem 39(8):1157–1162. https://doi.org/10.1007/s10800-008-9774-1
Obrovac MN, Krause LJ (2007) Reversible cycling of crystalline silicon powder. J Electrochem Soc 154(2):A103–A108. https://doi.org/10.1149/1.2402112
Pereira-Nabais C, Światowska J, Chagnes A, Ozanam F, Gohier A, Tran-Van P, Cojocaru C-S, Cassir M, Marcus P (2013) Interphase chemistry of Si electrodes used as anodes in Li-ion batteries. Appl Surf Sci 266:5–16. https://doi.org/10.1016/j.apsusc.2012.10.165
McCormac K, Byrd I, Brannen R, Seymour B, Li J, Wu J (2015) Preparation of porous Si and TiO2 nanofibres using a sulphur-templating method for lithium storage. Physica Status Solidi 212(4):877–881. https://doi.org/10.1002/pssa.201431834
Wang Z, Su Q, Deng H, He W, Lin J, Fu YQ (2014) Modelling and simulation of electron-rich effect on Li diffusion in group IVA elements (Si, Ge and Sn) for Li ion batteries. J Mater Chem A 2(34):13976–13982. https://doi.org/10.1039/C4TA01614A
Wu JJ, Bennett WR (2012) Fundamental investigation of Si anode in Li-Ion cells. In: 2012 IEEE Energytech, 29–31. pp 1–5. https://doi.org/10.1109/energytech.2012.6304667
Väli R, Jänes A, Lust E (2017) Alkali-metal insertion processes on nanospheric hard carbon electrodes: an electrochemical impedance spectroscopy study. J Electrochem Soc 164(11):E3429–E3437. https://doi.org/10.1149/2.0431711jes
Acknowledgements
This work is supported by National Science Foundation Division of Chemical, Bioengineering, Environmental and Transport Systems (NSF CBET Award #1800619). JW, CA, PB, and SX sincerely acknowledge the generous support provided by Georgia Southern University. C.J. and A.S. thank the support from State University of New York at Binghamton.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, J., Anderson, C., Beaupre, P. et al. Co-axial fibrous silicon asymmetric membranes for high-capacity lithium-ion battery anode. J Appl Electrochem 49, 1013–1025 (2019). https://doi.org/10.1007/s10800-019-01343-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-019-01343-w