Abstract
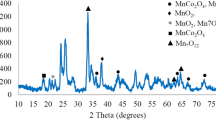
The awareness of energy, environment, and economy for electrolysis has required the development of new methods to prevent environmental pollution, such as the emission of acid fog, CO2, and SO2. Hence, the use of gas diffusion electrode as a cathode in the electrodeposition of manganese dioxide to save energy and protect the environment is attracting research interest. In this work, a gas diffusion electrode consisting of a mixture of Pt/TiO2-CNx nanocatalysts and two different additive conductor supports that activate carbon and acetylene black were synthesized. The Pt/TiO2-CNx nanocatalysts and Pt/TiO2-CNx gas diffusion electrode were characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, and X-ray photoelectron spectroscopy. The Pt/TiO2-CNx nanowire catalyst showed strong interaction between the Pt nanoparticles (~2.21 nm) and TiO2-CNx nanowire support. Results of electrochemical tests in 120 g of MnSO4 + 30 g of H2SO4 at 80 °C show that the Pt/TiO2-CNx gas diffusion electrode used in the electrodeposition of manganese dioxide could save electric energy by approximately 60%. Furthermore, the lifetime of the gas diffusion electrode of Pt/TiO2-CNx nanocatalysts was about twofold longer than that of the gas diffusion electrode of Pt/C and Pt/TiO2 nanocatalysts. The Pt/TiO2-CNx nanowire catalyst exhibited high anti-acid corrosion, activity, and stability, indicating its potential important application in the electrodeposition of manganese dioxide.
Graphical Abstract









Similar content being viewed by others
References
Li XL, Zhang YL, Zhong Q, Li TT, Li HY, Huang JM (2014) Appl Surf Sci 313:877
Donne SW, Kennedy JH (2004) J Appl Electrochem 34:159
Xu CN, Miyazaki K, Watanabe T (1998) Sensor Actuators B 46:87
Xiao F, Li YQ, Zan XL, Liao K, Xu R, Duan HW (2012) Adv Funct Mater 22:2487
He YM, Chen WJ, Li XD, Zhang ZX, Fu JC, Zhao CH, Xie EQ (2013) ACS NANO 7:174
Kumar VG, Gnanaraj JS, David SB, Pickup DM, Van-Eck ERH, Gedanke A, Aurbach D (2003) Chem Mater 15:4211
Qu DY (1999) J Appl Electrochem 29:511
Tang J, Meng HM, Huang LL (2014) RSC Adv 4:16512
Tang J, Meng HM, Li S, Yu MH, Li H, Shi JH (2015) Electrochim Acta 170:92
Bojdi MK, Behbahani M, Sahragard A, Amin BG, Fakhari A, Bagheri A (2014) Electrochim Acta 149:108
Bojdi MK, Behbahani M, Omidi F, Hesam G (2016) New J Chem 40:4519
Bojdi MK, Mashhadizadeh MH, Behbahani M, Farahani A, Davarani SSH, Bagheri A (2014) Electrochim Acta 136:59
Bojdi MK, Behbahani M, Najafi M, Bagheri A, Omidi F, Salimi S (2015) Electroanalysis 27:2458
Fan ZJ, Yan J, Wei T, Zhi LJ, Ning GQ, Li TY, Wei F (2011) Adv Funct Mater 21:2366
Hosseini H, Behbahani M, Mahyari M, Kazerooni H, Bagheri A, Shaabani A (2014) Biosens Bioelectron 59:412
Bojdi MK, Behbahani M, Mashhadizadeh MH, Bagheri A, Davarani SSH, Farahani A (2015) Mat Sci Eng C 48:213
Bojdi MK, Behbahani M, Hesam G, Mashhadizadeh MH (2016) RSC ADV 6:32374
Kadirgan F, Kannan AM, Atilan T, Beyhan S, Ozenler SS, Suzer S, Yörür A (2009) Int J Hydrog Energy 34:9450
Lia B, Higginsc DC, Xiao QF, Yang DJ, Zhng C, Cai M, Chen ZW, Ma JX (2015) Appl Catal B 162:133
Ioroi T, Akita T, Asahi M, Yamazaki SI, Siroma Z, Fujiwara N, Yasuda K (2013) J Power Sources 223:183
Kiros Y, Pirjamali M, Bursell M (2006) Electrochim Acta 51:3346
Chatenet M, Aurousseau M, Durand R, Andolfatto F (2003) J Electrochem Soc 150:D47
Chung S, Choun M, Jeong B, Lee J (2016) J Energy Chem 25:258
Lin KJ, Lu YX, Du SF, Li XY, Dong HS (2016) Int J Hydrog Energy 41:7622
Jia JC, Wang H, Ji S, Yang HJ, Li XS, Wang RF (2014) Electrochim Acta 141:13
Womack M, Vendan M, Molian P (2004) Appl Surf Sci 221:99
Li ZS, Li YY, Jiang SP, He GQ, Shen PK (2014) J Mater Chem A 2:16898
Easton EB, Astill TD, Holdcroft S (2005) J Electrochem Soc 152:A752
Bauer A, Lee K, Song CJ, Xie YS, Zhang JJ, Hui R (2010) J Power Sources 195:3105
Huang K, Sasaki K, Adzic RR, Xing YC (2012) J Mater Chem 22:16824
Jiang ZZ, Wang ZB, Chu YY, Gu DM, Yin GP (2011) Energy Environ Sci 4:728
Hillman AR, Dong QZ, Mohamoud MA, Efimov L (2010) Electrochim Acta 55:8142
Fan Y, Yang ZJ, Huang P, Zhang X, Liu YM (2013) Electrochim Acta 105:157
Li W, Bai Y, Li FJ, Liu C, Chan KY, Feng X, Lu XH (2012) J Mater Chem 22:4025
XiaoYH, Zhan GH, Fu ZG (2014) Electrochim Acta 141:279
Senevirathne K, Hui R, Campbell S, Ye S, Zhang J (2012) Electrochim Acta 59:538
Wu G, Chen YS, Xu BQ (2005) Electrochem Commun 7:1237
Li XG, Hsing IM (2006) Electrochim Acta 51:5250
Oh HS, Oh JG, Lee WH, Kim HJ, Kim H (2011) Int J Hydrog Energy 36:8181
Furuya N, Aikawa H (2000) Electrochim Acta 45:4251
Zhang H, Meng HM (2014) Sens Transducers 169:296
Tang Y, Li YJ, Sun YZ, Wang JX, Chen YM, Yang XJ, Wan PY (2013) Electrochem Commun 27:108
Gurau V, Bluemle MJ, Castro ESD, Tsou YM, Jr JAM, Jr TAZ (2006) J Power Sources 160:1156
Devennry M, Donne SW, Gorer S (2004) J Appl Electrochem 34:643
Acknowledgements
The authors acknowledge the financial support of the Natural Science Foundation of China (51274027).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, J., Meng, H. & He, Y. Energy-saving synthesis of electrolytic manganese dioxide using oxygen cathode with Pt/TiO2-CNx nanocatalysts. J Appl Electrochem 47, 653–659 (2017). https://doi.org/10.1007/s10800-017-1065-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-017-1065-2