Abstract
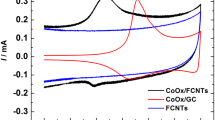
Oxygen reduction electrode is one of the important electrode materials for energy technologies because of the technical importance of oxygen reduction reaction (ORR) in the development of fuel cells and batteries. In this work, a fast, simple and applicable electrodeposition process has been used to modify the oxygenated functionalized carbon nanotubes (FCNTs) with cobalt oxide nanoparticles (CoOx) for use as an active catalyst electrode for ORR in alkaline medium. The prepared electrodes were characterized with structural techniques (XRD and TEM) to confirm the deposition of CoOx nanoparticle on FCNTs surface. The electrocatalytic activity of the prepared electrodes towards ORR was evaluated using different electrochemical methods such as cyclic voltammetry, linear sweep voltammetry combined with rotating disk electrode technique and chronoamperometry. Based on RDE measurements, the CoOx/FCNTs showed higher electrocatalytic activity and the mechanism of ORR proceeds via the four-electron mechanism, the favourable mechanism shown by the noble metal catalysts. However, the CoOx electrode exhibited only the two-electron mechanism with formation of hydrogen peroxide, rather than the four-electron mechanism, while the FCNTs electrode exhibited the two parallel mechanisms favouring four-electron mechanism only at higher overpotential. These results indicate the synergistic effect of the coupling between FCNTs and CoOx nanoparticles catalyzing the ORR via the direct four-electron mechanism. Such a synergistic effect is assumed to be attributed to the formation of an active interface between the FCNTs and CoOx resulting in highly catalytic active sites that allow the adsorption of oxygen and simultaneous reduction and/or the chemical decomposition of the intermediates, mainly the peroxide intermediate formed during the two-electron pathway mechanism. Moreover, compared to the benchmarked catalyst (Pt/C), the non-precious CoOx/FCNTs electrode showed both the higher stability under continuous ORR at a fixed potential for 6 h and higher tolerance for methanol poisoning. The results reported in this work could contribute to the development of high stable and fuel poisoning tolerance cathode electrodes for direct alkaline alcohol fuel cells.
Graphical Abstract






Similar content being viewed by others
References
Ge X, Sumboja A, Wuu D, An T, Li B, Goh FWT, Sum T, Hor TSA, Zong Y, Liu Z (2015) Oxygen reduction in alkaline media: from mechanisms to recent advances of catalysts. ACS Catal 5:4643–4667. doi:10.1021/acscatal.5b00524
Lee WJ, Maiti NU, Lee MJ, Lim J, Han HT, Kim SO (2014) Nitrogen-doped carbon nanotubes and graphene composite structures for energy and catalytic applications. Chem Commun 50:6818–6830. doi:10.1039/C4CC00146J
Yan J, Lu H, Huang Y, Fu J, Mo S, Wei C, Miao YE, Liu T (2015) Polydopamine-derived porous carbon fiber/cobalt composites for efficient oxygen reduction reaction. J Mater Chem A 3:1–9. doi:10.1039/C5TA06217A
Huang C, Li C, Shi G (2012) Graphene based catalysts. Energy Environ Sci 5:8848–8868. doi:10.1039/C2EE22238H
Yang Z, Nie H, Chen X, Chen X, Huang S (2013) Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction. J Power Sources 236:238–249. doi:10.1016/j.jpowsour.2013.02.057
Zhang L, Zhang J, Wilkinson DP, Wang H (2006) Progress in preparation of non-noble electrocatalysts for PEM fuel cell reactions. J Power Sources 156:171–182. doi:10.1016/j.jpowsour.2005.05.069
Zhang L, Xia Z (2011) Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J Phys Chem C 115:11170–11176. doi:10.1021/jp201991j
Matter PH, Zhang L, Ozkan US (2006) The role of nanostructure in nitrogen-containing carbon catalysts for the oxygen reduction reaction. J Catal 239:83–96. doi:10.1016/j.jcat.2006.01.022
Daems N, Sheng X, Vankelecom IFJ, Pescarmona PP (2014) Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J Mater Chem A 2:4085–4110. doi:10.1039/C3TA14043A
Zhang G, Lu W, Cao F, Xiao Z, Zheng X (2016) N-doped graphene coupled with Co nanoparticles as an efficient electrocatalyst for oxygen reduction in alkaline media. J Power Sources 302:114–125. doi:10.1016/j.jpowsour.2015.10.055
Zhong G, Wang H, Yu H, Peng F (2015) Nitrogen doped carbon nanotubes with encapsulated ferric carbide as excellent electrocatalyst for oxygen reduction reaction in acid and alkaline media. J Power Sources 286:495–503. doi:10.1016/j.jpowsour.2015.04.021
Yang H, Wang H, Ji S, Linkov V, Wang R (2014) Synergy between isolated-Fe3O4 nanoparticles and CNx layers derived from lysine to improve the catalytic activity for oxygen reduction reaction. Int J Hydrog Energy 39:3739–3745. doi:10.1016/j.ijhydene.2013.12.160
Ma Y, Wang H, Ji S, Goh J, Feng H, Wang R (2014) Highly active Vulcan carbon composite for oxygen reduction reaction in alkaline medium. Electrochim Acta 133:391–398. doi:10.1016/j.electacta.2014.04.080
Chung H, Won JH, Zelenay P (2013) Active and stable carbon nanotube/nanoparticle composite electrocatalyst for oxygen reduction. Nat Commun 4:1922. doi:10.1038/ncomms2944
Liang Y, Wang H, Diao P, Chang W, Hong G, Li Y, Gong M, Xie L, Zhou J, Wang J, Regier TZ, Wei F, Dai H (2012) Oxygen reduction electrocatalyst based on strongly coupled cobalt oxide nanocrystals and carbon nanotubes. Am Chem Soc 134:15849–15857. doi:10.1021/ja305623m
Xu J, Yu Q, Wu C, Guan L (2015) Oxygen reduction electrocatalysts based on spatially confined cobalt monoxide nanocrystals on holey N-doped carbon nanowires: the enlarged interfacial area for performance improvement. Mater Chem A 3:21647–21654. doi:10.1039/C5TA05757D
Wang S, Cui Z, Cao M (2015) A template-free method for preparation of cobalt nanoparticles embedded in N-doped carbon nanofibers with a hierarchical pore structure for oxygen reduction. Chem A Eur J 21:2165–2172. doi:10.1002/chem.201404884
Bezerra CWB, Zhang L, Lee K, Liu H, Marques ALB, Marques EP, Wang H, Zhang J (2008) A review of Fe–N/C and Co–N/C catalysts for the oxygen reduction reaction. Electrochim Acta 53:4937–4951. doi:10.1016/j.electacta.2008.02.012
Jaouen F, Proietti E, Lefèvre M, Chenitz R, Dodelet JP, Wu G, Chung HT, Johnston CM, Zelenay P (2011) Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells. Energy Environ Sci 4:114–130. doi:10.1039/C0EE00011F
Liu J, Jiang L, Tang Q, Zhang B, Su DS, Wang S, Sun G (2012) Coupling effect between cobalt oxides and carbon for oxygen reduction reaction. ChemSusChem 5:2315–2318. doi:10.1002/cssc.201200563
Liu J, Jiang L, Zhang B, Jin J, Su DS, Wang S, Sun G (2014) Controllable synthesis of cobalt monoxide nanoparticles and the size-dependent activity for oxygen reduction reaction. ACS Catal 4:2998–3001. doi:10.1021/cs500741s
Su Z, Zhu Y, Jiang H, Shen J, Yang X, Zou W, Chen J, Li C (2014) Cobalt nanoparticles embedded in N-doped carbon as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nanoscale 6:15080–15089. doi:10.1039/C4NR04357J
Herrmann I, Kramm UI, Fiechte S, Bogdanoff P (2009) Oxalate supported pyrolysis of CoTMPP as electrocatalysts for the oxygen reduction reaction. Electrochim Acta 54:4275–4287. doi:10.1016/j.electacta.2009.02.056
Xu P, Chen W, Wang Q, Zhu T, Wu M, Qiao J, Chen Z, Zhang J (2015) Effects of transition metal precursors (Co, Fe, Cu, Mn, or Ni) on pyrolyzed carbon supported metal-aminopyrine electrocatalysts for oxygen reduction reaction. RSC Adv 5:6195–6206. doi:10.1039/C4RA11643G
Hernandez-Fernandez P, Baranton S, Rojas S, Ocon P, Leger JM, Fierro JLG (2011) Insights into the effects of functional groups on carbon nanotubes for the electrooxidation of methanol. Langmuir 27:9621–9629. doi:10.1021/la2011452
Yang J, Zhang W, Gunasekaran S (2011) A low-potential, H2O2-assisted electrodeposition of cobalt oxide/hydroxide nanostructures onto vertically-aligned multi-walled carbon nanotube arrays for glucose sensing. Electrochim Acta 56:5538–5544. doi:10.1016/j.electacta.2011.03.087
Vashista SK, Zheng D, Al-Rubeaand K, Luonge JHT, Sheu FS (2011) Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol Adv 29:169–188. doi:10.1016/j.biotechadv.2010.10.002
Helia H, Pishahang J (2014) Cobalt oxide nanoparticles anchored to multiwalled carbon nanotubes: synthesis and application for enhanced electrocatalytic reaction and highly sensitive nonenzymatic detection of hydrogen peroxide. Electrochim Acta 123:518–526. doi:10.1016/j.electacta.2014.01.032
Wang Y, Zhang D, Liu H (2010) A study of the catalysis of cobalt hydroxide towards the oxygen reduction in alkaline media. J Power Sources 195:3135–3139. doi:10.1016/j.jpowsour.2009.11.112
Wu J, Zhang D, Wang Y, Wan Y, Hou B (2012) Catalytic activity of graphene–cobalt hydroxide composite for oxygen reduction reaction in alkaline media. J Power Source 198:122–126. doi:10.1016/j.jpowsour.2011.10.007
Nassr ABAA, Sinev I, Grünert W, Bron M (2013) PtNi supported on oxygen functionalized carbon nanotubes: in depth structural characterization and activity for methanol electrooxidation. Appl Catal B Environ 142–143:849–860. doi:10.1016/j.apcatb.2013.06.013
Davis RE, Horvath GL, Tobias CW (1967) The solubility and diffusion coefficient of oxygen in potassium hydroxide solutions. Electrochim Acta 12:287–297. doi:10.1016/0013-4686(67)80007-0
Xia W, Wang Y, Bergstra R, Kundu S, Muhler M (2007) Surface characterization of oxygen-functionalized multi-walled carbon nanotubes by high-resolution X-ray photoelectron spectroscopy and temperature-programmed desorption. Appl Surf Sci 254:247–250. doi:10.1016/j.apsusc.2007.07.120
Steimecke M, Rümmler S, Bron M (2015) The effect of rapid functionalization on the structural and electrochemical properties of high-purity carbon nanotubes. Electrochim Acta 163:1–8. doi:10.1016/j.electacta.2015.02.142
Yu J, Chen G, Sunarso J, Zhu Y, Ran R, Zhu Z, Zhou W, Zongping S (2016) Cobalt oxide and cobalt-graphitic carbon core–shell based catalysts with remarkably high oxygen reduction reaction activity. Adv Sci. doi:10.1002/advs.201600060
Heli H, Yadegari H (2010) Nanoflakes of the cobaltous oxide, CoO: synthesis and characterization. Electrochim Acta 55:2139–2148. doi:10.1016/j.electacta.2009.11.047
Kruusenberg I, Alexeyeva N, Tammeveski K (2009) The pH-dependence of oxygen reduction on multi-walled carbon nanotube modified glassy carbon electrodes. Carbon 47:651–658. doi:10.1016/j.carbon.2008.10.032
Kruusenberg I, Marandi M, Sammelselg V, Tammeveski K (2009) Hydrodynamic deposition of carbon nanotubes onto HOPG: the reduction of oxygen on CNT/HOPG electrodes in alkaline solution. Electrochem Solid State Lett 12:F31–F34. doi:10.1149/1.3202406
Zhong G, Wang H, Yu H, Wang H, Peng F (2016) Chemically drilling carbon nanotubes for electrocatalytic oxygen reduction reaction. Electrochim Acta 190:49–56. doi:10.1016/j.electacta.2015.12.216
Maa Z, Guoa C, Yina Y, Zhanga Y, Wua H, Chena C (2015) The use of cheap polyaniline and melamine co-modified carbon nanotubes as active and stable catalysts for oxygen reduction reaction in alkaline medium. Electrochim Acta 160:357–362. doi:10.1016/j.electacta.2015.02.053
Wang Y, Wang Z, Wu X, Liu X, Li M (2016) Synergistic effect between strongly coupled CoAl layered double hydroxides and graphene for the electrocatalytic reduction of oxygen. Electrochim Acta 192:196–204. doi:10.1016/j.electacta.2016.01.201
Demarconnay L, Coutanceau C, Léger JM (2008) Study of the oxygen electroreduction at nanostructured PtBi catalysts in alkaline medium. Electrochim Acta 53:3232–3241. doi:10.1016/j.electacta.2007.07.006
El-Deab MS, El-Nowihy GH, Mohammad AM (2015) Synergistic enhancement of the electro-oxidation of methanol at tailor-designed nanoparticle-based CoOx/MnOx/Pt ternary catalysts. Electrochim Acta 165:402–409. doi:10.1016/j.electacta.2015.02.231
Liu Y, Higgins DC, Wu J, Fowler M, Chen Z (2013) Cubic spinel cobalt oxide/multi-walled carbon nanotube composites as an efficient bifunctionalelectrocatalyst for oxygen reaction. Electrochem Commun 3:125–129. doi:10.1016/j.elecom.2013.05.035
Acknowledgements
A.Z. Al-Hakemy gratefully acknowledges the SRAT-City for the internship to carry out the experimental work of his master thesis in its laboratories. The authors are greatly thankful for Mr. Osama Khamis Ahmed for his help with XRD measurements. AB.A. Nassr acknowledges the financial support from German Academic Exchange Service (DAAD), Bonn, Germany, on the Grant for Small equipment. AB.A. Nassr acknowledges the great support and help from Prof. Michael Bron, Professor of Industrial Chemistry at Martin-Luther University Halle-Wittenberg, Halle (Saale), Germany, with materials and accessories that were necessary to achieve this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al-Hakemy, A.Z., Nassr, A.B.A.A., Naggar, A.H. et al. Electrodeposited cobalt oxide nanoparticles modified carbon nanotubes as a non-precious catalyst electrode for oxygen reduction reaction. J Appl Electrochem 47, 183–195 (2017). https://doi.org/10.1007/s10800-016-1027-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-016-1027-0