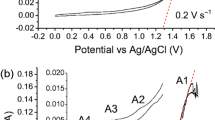
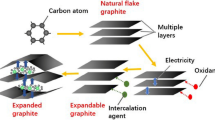
Abstract
The electrochemical oxidation of graphite matrix from the simulative fuel elements for high-temperature gas-cooled reactor was investigated experimentally using NaNO3 solution as an electrolyte. The intercalation and oxidation reactions of graphite were investigated by means of cyclic voltammetry. In addition, the morphological changes of the graphite anodes at predetermined intervals of time during the electro-oxidation process were examined by scanning electron microscopy. The structural transformation of graphite was systematically characterized by different methods. Results showed that the electro-oxidation process induced oxygen-containing groups (i.e., hydroxyl, epoxide, carbonyl/ketone, and carboxyl groups) into the graphite backbone. Electrolytic graphite oxide presented a heterogeneous, indeterminate, and disordered system composed of crystalline and amorphous phases. The structure and microstructure of nuclear graphite, particularly its cracks and defects, primarily determined its destruction pathway during the electrolytic process. The mechanism of graphite lattice destruction could be attributed to the complicated interplay of water electrolysis, anionic intercalation, and gas evolution. The mechanical force caused by gas eruptions among the graphite lattice is the most important and essential factor favoring disintegration.
Graphical Abstract












Similar content being viewed by others
References
Tang C, Tang Y, Zhu J, Zou Y, Li J, Ni X (2002) Design and manufacture of the fuel element for the 10 MW high temperature gas-cooled reactor. Nucl Eng Des 218:91–102
Sawa K, Ueta S (2004) Research and development on HTGR fuel in the HTTR project. Nucl Eng Des 233:163–172
Sawa K, Yoshimuta S, Shiozawa S (1998) Study on storage and reprocessing concept of the high temperature engineering test reactor (HTTR) fuel. IAEA-XA9848073 1043:177–189
Masson M, Grandjean S, Lacquement J, Bourg S, Delauzun JM, Lacombe J (2006) Block-type HTGR spent fuel processing: CEA investigation program and initial results. Nucl Eng Des 236:516–525
Ishihara M, Sumita J, Shibata T, Iyoku T, Oku T (2004) Principle design and data of graphite components. Nucl Eng Des 233:251–260
Zhou Z, Bouwman WG, Schut H, Pappas C (2014) Interpretation of X-ray diffraction patterns of (nuclear) graphite. Carbon 69:17–24
Merz E (1970) On the disintegration of graphite by the formation of electrolytic graphite intercalation compounds. Kerntechnik 12:341–346
Hoogen NG, Mer E (1983) Evaluation of potential head-end procedures for graphite-containing fuel elements. Nucl Technol 61:380–387
Tian L, Wen M, Li L, Chen J (2009) Disintegration of graphite matrix from the simulative high temperature gas-cooled reactor fuel element by electrochemical method. Electrochim Acta 54:7313–7317
Tian L, Wen M, Chen J (2010) Analysis of electrochemical disintegration process of graphite matrix. Electrochim Acta 56:985–989
Fritz JOBA (1983) The electrochemistry of black carbons. Angew Chem Int Ed 22:950–975
Beilby AL, Sasaki TA, Stern HM (1995) Electrochemical pretreatment of carbon electrodes as a function of potential, pH, and time. Anal Chem 67:976–980
Kevin JCBC, Hathcock W (1995) Incipient electrochemical oxidation of highly oriented pyrolytic graphite: correlation between surface blistering and electrolyte anion intercalation. Anal Chem 67:2201–2206
Abdelkader AM, Cooper AJ, Dryfe RAW, Kinloch IA (2015) How to get between the sheets: a review of recent works on the electrochemical exfoliation of graphene materials from bulk graphite. Nanoscale 7:6944–6956
Charles JCBE, Goss A (1993) Imaging the incipient electrochemical oxidation of highly oriented pyrolytic graphite. Anal Chem 65:1378–1389
Liu H, Xu Q, Yan C, Qiao Y (2011) Corrosion behavior of a positive graphite electrode in vanadium redox flow battery. Electrochim Acta 56:8783–8790
Fan X, Li Y, Wang S, Lu Y, Xu H, Liu J, Yan C (2015) Investigation on the pseudocapacitive characteristics of the electrochemically modified graphite electrode in electrolytes with different pH. Electrochim Acta 176:70–76
Alliata D, Ring PH, Haas O, Tz RK, Siegenthaler H (1999) Anion intercalation into highly oriented pyrolytic graphite studied by electrochemical atomic force microscopy. Electrochem Commun 1:5–9
Bordet F, Ahlbrecht K, Tübke J, Ufheil J, Hoes T, Oetken M, Holzapfel M (2015) Anion intercalation into graphite from a sodium-containing electrolyte. Electrochim Acta 174:1317–1323
Wang P, Contescu CI, Yu S, Burchell TD (2012) Pore structure development in oxidized IG-110 nuclear graphite. J Nucl Mater 430:229–238
Lee DW, De Los Santos V L, Seo JW, Felix LL, Bustamante D A, Cole JM, Barnes CHW (2010) The structure of graphite oxide: investigation of its surface chemical groups. J Phys Chem B 114:5723–5728
Jeong H, Lee YP, Lahaye RJWE, Park M, An KH, Kim IJ, Yang C, Park CY, Ruoff RS, Lee YH (2008) Evidence of graphitic AB stacking order of graphite oxides. J Am Chem Soc 130:1362–1366
Afanasov IM, Shornikova ON, Kirilenko D, Vlasov II, Zhang L, Verbeeck J, Avdeev VV, Van Tendeloo G (2010) Graphite structural transformations during intercalation by HNO3 and exfoliation. Carbon 48:1862–1865
Iwashita N, Park CR, Fujimoto H, Shiraishi M, Inagaki M (2004) Specification for a standard procedure of X-ray diffraction measurements on carbon materials. Carbon 42:701–714
Zhan D, Ni Z, Chen W, Sun L, Luo Z, Lai L, Yu T, Wee ATS, Shen Z (2011) Electronic structure of graphite oxide and thermally reduced graphite oxide. Carbon 49:1362–1366
Casabianca LB, Shaibat MA, Cai WW, Park S, Piner R, Ruoff RS, Ishii Y (2010) NMR-based structural modeling of graphite oxide using multidimensional 13C solid-state NMR and ab initio chemical shift calculations. J Am Chem Soc 132:5672–5676
Parvez K, Wu Z, Li R, Liu X, Graf R, Feng X, Müllen K (2014) Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J Am Chem Soc 136:6083–6091
He HY, Riedl T, Lerf A, Klinowski J (1996) Solid-state NMR studies of the structure of graphite oxide. J Phys Chem 100:19954–19958
Ferrari AC, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B 61:14095–14107
Boukhvalov DW, Katsnelson MI (2008) Modeling of graphite oxide. J Am Chem Soc 130:10697–10701
Weiwei C, Piner RD, Stadermann FJ, Sungjin P, Shaibat MA, Ishii Y, Dongxing Y, Velamakanni A, Sung JA, Stoller M, Jinho A, Dongmin C, Ruoff RS (2008) Synthesis and solid-state NMR structural characterization of 13C-labeled graphite oxide. Science 321:1815–1817
Beck F, Jiang J, Krohn H (1995) Potential oscillations during galvanostatic overoxidation of graphite in aqueous sulphuric acids. J Electroanal Chem 389:161–165
Rueffer M, Bejan D, Bunce NJ (2011) Graphite: an active or an inactive anode? Electrochim Acta 56:2246–2253
Robert Bowling RTPA (1989) Mechanism of electrochemical activation of carbon electrodes: role of graphite lattice defects. Langmuir 5:683–688
McDermott MT, Kneten K, McCreery RL (1992) Anthraquinonedisulfonate adsorption, electron-transfer kinetics, and capacitance on ordered graphite electrodes: the important role of surface defects. J Phys Chem 96:3124–3130
Freund MS, Brajtertoth A, Cotton TM, Henderson ER (1991) Scanning tunneling microscopy and atomic force microscopy in the characterization of activated graphite electrodes. Anal Chem 63:1047–1049
Alsmeyer DC, McCreery ARL (1991) In situ Raman monitoring of electrochemical graphite intercalation and lattice damage in mild aqueous acids. Anal Chem 64:1528–1533
Low CTJ, Walsh FC, Chakrabarti MH, Hashim MA, Hussain MA (2013) Electrochemical approaches to the production of graphene flakes and their potential applications. Carbon 54:1–21
Acknowledgments
This work was supported by National Science and Technology Major Project (2014ZX06901-016) and Program for Changjiang Scholars and Innovative Research Team in University (IRT13026).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, G., Wen, M., Wang, S. et al. Insights into electrochemical behavior and anodic oxidation processing of graphite matrix in aqueous solutions of sodium nitrate. J Appl Electrochem 46, 1163–1176 (2016). https://doi.org/10.1007/s10800-016-0999-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-016-0999-0