Abstract
Investigations of the influence of electrolysis parameters such as the concentration of metal ammonia complexes, working electrode potential and temperature on the composition, structure and catalytic activity of synthesized alloys for water molecule reduction reaction in 2 M NaOH (T = 25 °C) were conducted. The alloys were deposited under potentiostatic conditions within potential range from −0.7 to −1.1 V in electrolytes of pH 9.5, containing ammonia complexes of cobalt(III) and palladium(II), [Co(NH3)6]3+ and [Pd(NH3)4]2+, of different concentration ratio. Structural changes in electrodeposited alloys were discussed based on results of X-ray diffraction measurements. An elemental analysis was performed using the energy-dispersive X-ray spectroscopy technique. Finally, based on results of galvanostatic measurements, the Tafel slope within the range of activation control for hydrogen evolution reaction was determined and mechanism of the process was discussed. The alloys presented low Tafel slope value, from 25.4 to 88.7 mV dec−1. The alloy of the highest activity for hydrogen evolution reaction contained 31.2 at.% of Pd.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Palladium and its alloys are a subject of interest in many research centres all over the world. This is mainly a result of palladium’s high catalytic activity and its lower price in comparison with platinum. Due to the price and low accessibility of Pd, intensive investigations are conducted, with the aim of creating alloys with catalytic properties comparable to those of palladium, but much cheaper than is element itself. Alloys of Co–Pd, Pd–Fe, Pd–Ni and Pd–Cr exhibit high catalytic activity for the four-electron oxygen reduction reaction (ORR) [1]. Properties of Co–Pd alloys for the reaction are close to the properties of pure platinum [2, 3]. This means that they can be used, for example, in direct methanol fuel cells (DMFC) and proton exchange membrane fuel cells [4, 5]. They show high tolerance for the influence of methanol used in DMFCs [6, 7]. Tests performed so far have demonstrated that Pd–Co alloys have much better electrocatalytic properties for ORR than does pure palladium [8]. In catalysers composed of two metals, of which one (Co) has partially occupied d orbitals, and the second (Pd) has d orbitals that are completely filled, the result of their interactions might be to lower Gibbs free energy for the process of electron transfer [9]. The overlapping orbitals may lead to displacement of the d orbital centre, and this displacement may cause changes in the surface activity of Pd. Formation of Co–Pd alloys also causes lattice compression, by shortening lengths of bonds between the metals [10]. This is the result of the previously mentioned overlapping of the orbitals, and of modifications to the electronic structure. Consequently, one can assume that the examined Co–Pd alloys will feature a high catalytic activity for the water molecule reduction reaction leading to the formation of gaseous hydrogen.
The composition of the alloy coatings obtained through electrolysis depends on many factors, including, among others, concentration of individual electrolyte components, the presence of complexing compounds, pH, temperature, substrate material (due to different overvoltage of the ions reduction of individual metals or their complexes), the potential of the working electrode and the value of the intensity of cathodic current applied. Conditions for the deposition of Co–Pd alloys were based on previous investigations [11–13], which included a thermodynamic analysis of the electrolyte, spectrophotometric tests identifying and defining the stability of metal complexes present in the electrolyte, voltammetric tests using electrochemical quartz crystal microbalance and spectroelectrochemical tests. These investigations enabled selection of the electrolyte pH, concentration of the complexing agent and concentrations of individual metals ions, thus ensuring the stability of the complexes present in the solution. Also, in these papers, the mechanism of electrode reactions accompanying co-reduction of [Pd(NH3)4]2+ and [Co(NH3)6]3+ complexes was discussed. The results lead to the conclusion that co-deposition of Pd and Co metals takes place as a result of a two-step reduction reaction of hexaamminecobalt(III) (1) and a one-step reduction reaction of tetraamminepalladium(II) (2) [13].
In the literature, there are no works devoted to the electrochemical synthesis of Co–Pd alloys from solutions containing ammonia complexes of Co(III) and Pd(II). The present work demonstrates the results of tests concerning the deposition of Co–Pd alloys through co-reduction of [Co(NH3)6]3+ and [Pd(NH3)4]2+, and their properties, including electrocatalytic ones.
2 Experimental details
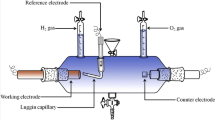
The electrolyte was prepared by dissolving Co(NH3)6Cl3 (Across Organics), Pd(NH3)4Cl2·H2O (Alfa Aesar) and NH4Cl (POCH) (1.62 M) (analytical grade reagents) in deionized water. The alloys were potentiostatically deposited from electrolytes of [Co(NH3)6]3+ concentration range from 5 to 50 mM and constant [Pd(NH3)4]2+ concentration of 10 mM. The pH of the electrolyte was adjusted by the addition of NaOH (9.5). Tests were performed in a Teflon electrochemical cell. The working electrode (WE) was Cu substrate. The counter electrode (CE) was platinum sheet (Pt). The working electrode was placed at the bottom of the cell. The Ag/AgCl (3 M KCl) electrode was used as the reference electrode in deposition process as well as in catalytic activity measurements. The measurements were conducted at constant temperature of 298 K. Elemental analysis was performed using the EDS technique (Hitachi Su–70). Phase analysis was performed using the XRD method (Rigaku MiniFlex II). Electrocatalytic activity was measured in 2 M NaOH (T = 298 K) under galvanostatic conditions. All electrochemical measurements were performed with the use of potentiostat/galvanostat AutoLab PGSTAT30. The ohmic drop was corrected with the use of current-interruption method. It should be mentioned that cobalt(II) hydroxide layer present on the surface of the deposit after electrolysis was removed by water flux.
3 Results and discussion
3.1 Alloys composition
The tests began with the deposition of alloys from the electrolytes of different ratio of individual metals ions concentrations. Electrolysis was conducted for 1 h in the range of potentials from E = −0.7 to −1.1 V. The potential was changed every E = 0.1 V. Next, the elemental composition of cathodic deposits was analysed using the EDS technique. Simultaneously, reduction of Pd(II) and Co(III) complexes occurred within the range of potentials in which reduction of water molecule takes place due to the alkaline environment:
A sequence of the reaction is local alkalization of the near electrode area and transition into the range of thermodynamic stability of Co(OH)2. It causes the deposition of hydroxide phase on the electrode surface. Figure 1 shows content changes of individual components of an alloy depending on both the potential of working electrode and the ratio of complexes present in the electrolyte (T = 298 K). The content of Pd in the deposit was found to be between 20.95 and 78.0 at.%. (Fig. 1a). A decrease in the value of the concentration ratio [[Co(NH3)6]3+]/[[Pd(NH3)4]2+] was accompanied by an increase in Pd content in the alloy, whereas cobalt content declined. Additionally, it can be seen that in the case of electrolytes containing 25 and 50 mM of [Co(NH3)6]3+, with the decreasing the working electrode potential, Pd content also decreases. An increase in Pd content in deposits was observed in each case, at the potential of E = −1.0 V. A decrease in Co content in alloy coatings is probably caused by an increased reduction rate of water molecule, resulting in alkalization of the near electrode area. It results in an exchange of NH3 ligands to the hydroxyl group by cobalt(II) complexes and the precipitation of cobalt(II) hydroxide. The depletion of the near electrode area by electroactive cobalt complexes caused [Pd(NH3)4]2+ to be the main depolarizer in the electrode reaction, apart from the water molecule.
Similar tests were performed in order to determine the influence of electrolysis temperature on the composition of synthesized alloys. Electrolysis was performed at 25, 50 and 75 °C. Content changes of the alloys components, depending on deposition conditions, are presented in Fig. 2. Increasing the temperature 25–50 °C for E = − 0.7 V caused a significant decrease in Pd content in alloys from 43.82 to 31.27 at.% and an increase in Co content, while the increase in temperature to 75 °C at the same potential value results in an increase in Pd content to 52.81 at.%. Raising the temperature from 50 to 75 °C caused no significant changes of the alloys composition within potential range lower than −0.8 V. For remaining potential values, the increase in temperature results in an increase in Pd content in comparison with alloys deposited at 25 °C.
3.2 XRD analysis
The XRD technique was used to carry out structural and phase analysis of the alloys. Diffractograms of alloys deposited from electrolytes of different concentration ratios and different potentials of electrolysis at T = 25 °C are shown in Fig. 3. Peaks coming from Cu substrate and CoPd x alloy (phase α-Co–Pd and ε-Co–Pd) were identified on the diffractograms. On diffractograms registered at E = −0.7 V and with a decrease in [[Co(NH3)6]3+] concentration in the electrolyte, a peak coming from planes (111) and (200) of FCC α-Co–Pd phase as well as the peak coming from the (002) planes of hexagonal ε-Co–Pd phase are visible [14]. Additionally, displacement in the location of peaks coming from α-Co–Pd phase into the range of lower values 2Θ confirms an increase in Pd participation in the phase [15, 16]. Lowering the working electrode potential caused peaks from α-Co–Pd phase to move into the higher angle range, which indicates an increase in Co content in this phase. At concentrations [[Co(NH3)6]3+]/[[Pd(NH3)4]2+] = 50 mM/10 mM and for E = −0.9 and−1.0 V, there are two visible peaks coming from planes (100) and (002) of the ε-Co–Pd phase of hexagonal structure, while at remaining potentials, only peaks coming from (002) planes are visible. A decrease in the intensity of (111) peak on the diffractograms registered for alloys deposited at E = −0.7 V (coming from the Cu substrate), together with a decrease in the [[Co(NH3)6]3+]/[[Pd(NH3)4]2+] ratio and an increase in the intensity of (111) peak (coming from the α-Co–Pd phase), indicates an acceleration of the reduction reaction of [Pd(NH3)4]2+ by lowering of [Co(NH3)6]3+ concentration. Therefore, it shows that ammonia–cobalt(III) complexes have an inhibiting influence on the rate of reaction (2), and the complexes affect the value of the overpotential of tetraamminepalladium(II) complexes reduction. Displacement of the cathodic current maximum, connected with a reduction of [Pd(NH3)4]2+ complexes, towards lower potentials with an increase in [Co(NH3)6]3+ concentration was also observed on voltammograms presented in the work of Mech and co-workers [13]. A significant increase in the intensity of Cu substrate peaks was noted on diffractograms registered for alloys deposited at E = −1.0 and −1.1 V from electrolytes containing 10 and 5 mM of hexaamminecobalt(III). It results from a decrease in current efficiency connected with an increase in Pd content in the alloy. It indicates high catalytic activity for water molecule reduction reaction, which in turn results directly in an increase in charge consumed by the reaction and decrease in the cathodic deposit thickness.
Figure 4 presents diffractograms of alloys deposited at higher temperatures, i.e. 50 and 75 °C. An electrolyte with a ratio of [[Co(NH3)6]3+]/[[Pd(NH3)4]2+] = 50 mM/10 mM was applied in order to test the influence of temperature on structural changes in deposited alloys. Increased temperature of the electrolysis favoured the formation of ε-Co–Pd phase. The intensity of reflexes coming from (002) planes, for alloys deposited at T = 50 °C, increased continuously as the electrolysis potential decreased. This increase was not observed for alloys deposited at T = 75 °C. A significant lowering of electrolysis efficiency caused either by cobalt(II) hydroxide precipitation or hydrogen bubbles breaking off cathodic deposit could possibly explain this fact. In the case of alloys deposited at higher temperatures, an additional reflex coming from planes (101) of ε-Co–Pd phase was also observed.
3.3 Catalytic activity measurements
The electrocatalytic properties of the obtained alloys were also tested. Overpotential of hydrogen evolution on the alloys was determined based on the dependence (4):
where E i potential of electrode polarized by electric current flow (defined in galvanostatic measurements (t = 120 s) with the use of potentiostat/galvanostat Autolab PGSTAT30 in 2 M NaOH at 25 °C) and E s stationary potential (registered for electrode not polarized after t = 240 s). The Tafel slope was determined within the activation control range with the use of Tafel’s equation
where
R gas constant (8.314 J mol−1 K−1), T temperature (K), α transfer coefficient and F Faraday’s constant (96,485 C mol−1).
In the alkaline environment, the overall process of hydrogen evolution is described by two reactions [17–19]:
and
Theoretical values of the Tafel slope for the electrode process controlled by reactions either (7) or (8) can be found in Table 1. The values were calculated with the assumption that the symmetry coefficient β = 0.5 for temperature of 298 K with the use of electron transfer coefficient (α) for multistep process [20].
Therefore, conclusions about RDS can be drawn by testing the Tafel slope. If the Tafel slope is ca. 118 mV dec−1, the slowest step of the hydrogen evolution process is the reduction of water molecule. For a slope of ca. 30 mV dec−1, the slowest step is a recombination of hydrogen atoms adsorbed on the metal surface.
Figures 5 and 6 show Tafel dependencies registered within the range of activation control of water molecule reduction reaction for the alloys deposited from electrolytes of different ratios of concentrations [[Co(NH3)6]3+]/[[Pd(NH3)4]2+] at different temperatures. The lowest Tafel slope value (25.4 mV dec−1) was found for the alloy deposited at T = 50 °C, from an electrolyte containing 50 mM of [Co(NH3)6]3+ at E = −0.7 V (Fig. 6). The alloy contained 31.2 at.% of Pd. The Tafel slope value indicates that recombination of adsorbed hydrogen atoms is the slowest step in the process of hydrogen evolution. Cavalotti et al. also investigated electrocatalytic properties of Co–Pd alloys for HER (0.1 M NaOH) . The value of Tafel’s slope that they registered for electrochemically deposited Co–Pd alloy is 81 mV dec−1. The alloy of 50 nm thickness contained 80 at.% of Pd and was synthesized at galvanostatic conditions (10 mA cm−2, T = 60 °C) from the solution based on ammonia complexes. Resulted alloys independently of applied conditions (beside an alloy synthesized at 50 °C (E = −0.9 V)) possesses definitely lower values of Tafel’s slope than reported by Cavalotti and co-workers [21].
Equally high catalytic activity appears at low Tafel slope values. Values of (<30 mV dec−1) exhibit Co–31.2Pd (25.4 mV dec−1), Co–22.11Pd (27.4 mV dec−1), Co–23.95Pd (27.7 mV dec−1) and Co–28.16Pd (29.7 mV dec−1) alloys. Allowing for estimated margin of standard error in our measurements, one can conclude that Tafel slope values lower than 30 mV dec−1 can exhibit Co–53.45Pd (30.3 mV dec−1), Co–22.93Pd (30.3 mV dec−1), Co–60.89Pd (30.8 mV dec−1) and Co–70.64Pd (30.3 mV dec−1) alloys. It should be noted that the best catalytic activity exhibit Co–31.2Pd (25.4 mV dec−1), Co–22.11Pd (27.4 mV dec−1) and Co–23.95Pd (27.7 mV dec−1) alloys, deposited from electrolytes of [[Co(NH3)6]3+]/[[Pd(NH3)4]2+] = 50 mM/10 mM, which contained relatively low Pd concentration. Observations of Tafel slope changes for particular alloys show that electrolysis conditions have a significant influence on changes in the catalytic activity of the alloys.
4 Conclusions
The experiments indicate the possibility of obtaining Co–Pd alloys through electrolysis performed under potentiostatic conditions using solutions containing ammonia–cobalt(III) and palladium(II) complexes. Although electrolysis conditions were based on thorough tests conducted previously, it was not possible to obtain alloys without the presence of Co(OH)2 on the working electrode surface. The presence of Co(OH)2 was confirmed by EDS analysis. Obtained alloys were bright and well adhered. The alloys presented low Tafel slope value, in the range of from 25.4 to 88.7 mV dec−1. The alloy of the highest activity for HER contained 31.2 at.% Pd. An increase in temperature caused an increased hydroxide phase present on the surface of the deposits. The main factor enabling control of the composition of the coatings was the relation between the concentration of cobalt(III) and palladium(II) complexes. A change of electrolysis parameters considerably affected structural changes in the examined alloys, which was demonstrated on the registered XRD spectra.
References
Zhang L, Lee K, Zhang J (2007) Effect of synthetic reducing agents on morphology and ORR activity of carbon-supported nano-Pd–Co alloy electrocatalysts. Electrochim Acta 52:7964–7971
Savadogo O, Lee K, Oishi K, Mitsushima S, Kamiya N, Ota KI (2004) New palladium alloys catalyst for the oxygen reduction reaction in an acid medium. Electrochem Commun 6:105–109
Liu H, Li W, Manthiram A (2009) Factors influencing the electrocatalytic activity of Pd100-xCox (0 ≤ x ≥ 50) nanoalloys for oxygen reduction reaction in fuel cells. Appl Catal B 90:184–194
Noto VD, Negro E, Lavina S, Gross S, Pace G (2007) Pd–Co carbon-nitride electrocatalysts for polymer electrolyte fuel cells. Electrochim Acta 53:1606–1617
Zuluga S, Stolbov S (2011) Factors controlling the energetics of the oxygen reduction reaction on the Pd–Co electro-catalysts: insight from first principles. J Phys Chem 135:134702
Wang W, Zheng D, Du C, Zou Z, Zhang X, Xia B, Yang H, Akins DL (2007) Carbon-supported Pd–Co bimetallic nanoparticles as electrocatalysts for the oxygen reduction reaction. J Power Sources 167:243–249
Shao MH, Huang T, Liu P, Zhang J, Sasaki K, Vukmirovic MB, Adzic RR (2006) Palladium monolayer and palladium alloy electrocatalysts for oxygen reduction. Langmuir 22:10409–10415
Suo Y, Zhuang L, Lu J (2007) First-principles considerations in the design of Pd-alloy catalysts for oxygen reduction. Angew Chem Int 46:2862–2864
Wang YX, Balbuena PB (2005) Design of oxygen reduction bimetallic catalysts: ab initio-derived thermodynamic guidelines. J Phys Chem B 109:18902
Hammer B, Norskov JK (2000) Theoretical surface science and catalysis—calculations and concepts. Adv Catal 45:71–129
Mech K, Żabiński P, Kowalik R, Fitzner K (2012) Voltammetric study of electro-reduction of tetraamminepalladium(II) onto gold electrode. J Electroanal Chem 685:15–20
Mech K, Żabiński P, Kowalik R, Fitzner K (2012) EQCM, SEC and voltammetric study of kinetics and mechanism of hexaamminecobalt(III) electro-reduction onto gold electrode. Electrochim Acta 81:254–259
Żabiński P, Mech K, Kowalik R (2013) Analysis of Co–Pd alloys deposition from electrolytes based on [Co(NH3)6]3 + and [Pd(NH3)4]2 + complexes. Electrochim Acta 104:468–473
Ishida K, Nishizawa T (1991) The Co–Pd (Cobalt–Palladium) System. J Phase Equilib 12(1):83–87
Matsuo Y (1972) Ordered Alloys in Cobalt-Palladium System. J Phys Soc Jpn 32(4):):972. doi:10.1143/Jpsj.32.972
Heibel M, Kumar G, Wyse C, Bukovec P, Bocarsly AB (1996) Use of sol-gel chemistry for the preparation of cyanogels as ceramic and alloy precursors. Chem Mater 8(7):1504–1511. doi:10.1021/Cm960105u
Bockris JOM, Potter EC (1952) The mechanism of hydrogen evolution reaction. J Electrochem Soc 99:169–186
Mech K, Zabinski P, Mucha M, Kowalik R (2013) Electrodeposition of catalytically active Ni–Mo alloys. Arch Met Mat 58:227–229
Zabinski P, Mech K, Kowalik R (2013) Electrocatalically active Co–W and Co–W–C alloys electrodeposited in a magnetic field. Electrochim Acta 104:542–548
Noel M, Vasu KI (1990) Cyclic voltammetry and the frontiers of electrochemistry. Oxford & IBH Publishing Co. Pvt. Ltd., New Delhi
Bozzini B, Zangari G, Cavalotti PL (1994) Electrolytic hydrogen evolution on Co-alloy thin films prepared by electrochemical and autocatalytic deposition techniques. Electrochim Acta 39:1793–1887
Acknowledgments
This work was supported by the Polish National Center of Science under grants 2011/01/N/ST5/05509 and 2011/01/D/ST5/05743.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mech, K., Żabiński, P., Kowalik, R. et al. Electrodeposition of Co–Pd alloys from ammonia solutions and their catalytic activity for hydrogen evolution reaction. J Appl Electrochem 44, 97–103 (2014). https://doi.org/10.1007/s10800-013-0605-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-013-0605-7