Abstract
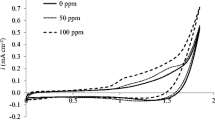
Oxalic acid is one of the proposed metabolites of the anodic oxidation of more complex organic molecules. In spite of its simple structure, its mineralization strongly depends on the nature of the electrode material at which the process is carried out. Sargisyan and Vasil’ev (Elektrokhimiya 18:845, 1982) pointed out such dependence, investigating the kinetic behavior of OA at different metal (Rh, Pd, Os, Ir, Pt and Au), at dimensionally stable anodes (RuO2–TiO2) and at glassy carbon (GC) electrodes. Their conclusions highlighted the important role played by the organic anion adsorption step, claiming that OA is oxidized with increasing difficulty at electrode materials having higher oxygen affinity. More recently, these assumptions have been supported by data on OA oxidation at high anodic potentials (Martínez-Huitle et al., Electrochim Acta 49:4027, 2004). To further enrich the picture, in the present paper, kinetic investigations were carried out at different mixed-oxides, Pt, GC and highly conductive, boron-doped diamond (BDD) electrodes, with either oxygen or fluorine at their surface.















Similar content being viewed by others
References
Sargisyan SA, Vasil’ev YuB (1982) Elektrokhimiya 18:845
Martínez-Huitle CA, Ferro S, De Battisti A (2004) Electrochim Acta 49:4027
Smirnova NV, Tsirlina GA, Pron’kin SN, Petrii OA (1999) Russ J Electrochem 35:113
Giner J (1961) Electrochim Acta 4:42
El Wakkad SES, Khalafalla SE, Shams El Din AM (1958) Egypt J Chem 1:23
Johnson JW, Wroblowa H, Bockris JO’M (1964) Electrochim Acta 9:639
Johnson JW, Mueller SC, James WJ (1971) Trans Faraday Soc 67:2167
Horanyi G (1974) J Electroanal Chem 51:163
Inzelt G, Szetey E (1981) Acta Chim Acad Sci Hung 3:269
YuB Vasil’ev, Sargisyan SA (1986) Electrochim Acta 31:645
Chollier-Brym MJ, Epron F, Lamy-Pitara E, Barbier J (1999) Catal Today 48:291
Gandini D, Mahé E, Michaud P-A, Haenni W, Perret A, Comninellis Ch (2000) J Appl Electrochem 30:1345
Sargisyan SA, Vasil’ev YuB (1982) Elektrokhimiya 18:954
Chollier-Brym MJ, Epron F, Lamy-Pitara E, Barbier J (1999) J Electroanal Chem 474:147
Sargisyan SA, Vasil’ev YuB (1982) Elektrokhimiya 18:961
Horanyi G, Hegedüs D, Rizmayer EM (1972) J Electroanal Chem 40:393
Pron’kin SN, Petrii OA, Tsirlina GA, Schiffrin DJ (2000) J Electroanal Chem 480:112
Horanyi G, Vertes G, Hegedüs D (1973) Acta Chim Hung 79:301
Smirnova NV, Petrii OA, Grzejdziak A (1988) J Electroanal Chem 251:73
Orts JM, Feliu JM, Aldaz A, Clavilier J, Rodes A (1990) J Electroanal Chem 281:73
Orts JM, Feliu JM, Aldaz A, Clavilier J, Rodes A (1990) J Electroanal Chem 281:199
Horanyi G (1980) Electrochim Acta 25:43
Horanyi G, Rizmayer EM, Inzelt G (1978) J Electroanal Chem 93:183
Casella IG (1999) Electrochim Acta 44:3353
Morozova NB, Shcheblykina GE, Vvedenskii AV (1999) Russ J Electrochem 35:310
Albalat R, Gomez E, Sarret M, Valles E (1989) Monatsh Chem 120:651
Alaune Z, Mazeikiene R (1987) Liet TSR Mokslu Akad Darb Ser B 2:11
Bock C, Smith A, MacDougall B (2002) Electrochim Acta 48:57
Obmornov EV, Karetnik VG, Koptelov VI, Dosovitskaya NA, Koptelova ZP, Masalova GP, Dosovitskii EI, Ostrovskaya VN (1967) GB Patent 1,095,100; (1970) US Patent 3,531,520
Obmornov EV, Karetnik VG, Koptelov VI, Dosovitskaya NA, Koptelova ZP, Masalova GP, Dosovitskii EI, Ostrovskaya VN (1970) US Patent 3,531,520
Pogodin VA, Kaverin NI, Krutova VP, Brazhnikov VA (1993) SU Patent 1,806,127
Stucki S, Koetz R, Carcer B, Suter W (1991) J Appl Electrochem 21:99
Scialdone O, Randazzo S, Galia A, Filardo G (2009) Electrochim Acta 54:1210
Martinez-Huitle CA, Ferro S (2006) Chem Soc Rev 35:1324
Ferro S, De Battisti A (2003) J Phys Chem B 107:7567
Ferro S, Dal Colle M, De Battisti A (2005) Carbon 43:1191
Morozov A, Ferro S, Martelli GN, De Battisti A (2005) WO 2005/014885, A1
Konoshita K (1982) In: Conway BE, Bockris JO’M, White RE (eds) Modern aspects of electrochemistry, vol 14. Plenum Press, New York, p 557
Murakami Y, Ohkawauchi H, Ito M, Yahikozawa K, Takasu Y (1994) Electrochim Acta 39:2551
Duo I, Fujishima A, Ch Comninellis (2003) Electrochem Comm 5:695
Gialdini C (1910) Gaz Chim Ital 38:485
Kruszyna HG, Bodek I, Libby LK, Milburn RM (1974) Inorg Chem 13:434
Kabir-ud-Din, Fatma W, Khan Z (2004) Colloids Surf A 234:159
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferro, S., Martínez-Huitle, C.A. & De Battisti, A. Electroxidation of oxalic acid at different electrode materials. J Appl Electrochem 40, 1779–1787 (2010). https://doi.org/10.1007/s10800-010-0113-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-010-0113-y