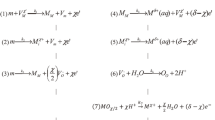Abstract
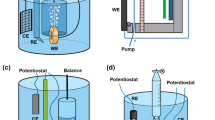
Quantitative characterization of surface reaction species improves understanding of reaction mechanisms, especially for heterogeneous reactions such as in corrosion and electrolysis. A universal mesh-capped surface pH probe was developed in order to investigate surface proton concentrations for aqueous metal dissolution/deposition reactions. This technique can be generally applied to characterize the surface pH either for bare metal reactions or metals corroding beneath a corrosion product film. Measurements with the new probe design have shown a good degree of reproducibility. The surface pH measurements during corrosion of mild steel have shown that a higher surface pH is achieved at the steel surface as compared with that of the bulk solution, just as predicted from theory. Chemical buffering effects on surface pH were observed during corrosion by contrasting the measurements obtained in HCl deaerated solutions with those containing CO2, i.e., carbonic acid (H2CO3) and acetic acid (CH3COOH/HAc) solutions. It was found that the surface pH was mainly controlled by the water chemistry and chemical buffer capacity. The surface pH is affected by the deposited corrosion scale porosity. A higher deviation of surface pH compared to the bulk value was observed for more dense layers with lower porosity.












Similar content being viewed by others
References
Han J, Yang Y, Brown B et al (2007) Corrosion/2007, paper no. 07323, Houston, TX
Han J, Yang Y, Brown B et al (2007) Corrosion/2008, paper no 08332, Houston, TX
Han J, Young D, Nešić S et al (2008) ICC/2008, paper no. 2511
Han J, Young D, Colijn H et al (2009) Ind Eng Chem Res 48(13):6296
Nordsveen M, Nešić S, Nyborg R et al (2003) Corrosion 59(5):443
Nešić S, Nyborg R, Stangeland A (2003) Corrosion 59(6):489
Nešić S, Lee K-LJ (2003) Corrosion 59(7):616
Dexter SC, Lin SH (1992) Corrosion 48(1):50
Yoshinobu T, Iwasaki H, Nakao M et al (1998) Jpn J Appl Phys Part 2 37(3B):L353
Yoshinobu T, Harada T, Iwasaki H (2000) Jpn J Appl Phys Part 2 39(4A):L318
King F, Litke CD, Tang Y (1995) J Electroanal Chem 384:105
Dražić DM, Djordjević S, Vojnović M (1969) 20th CITCE meeting, Strasburg
Lewandowski Z, Lee WC, Charazklis WG et al (1989) Corrosion 45(2):92
Wei C, Bard AJ, Nagy G et al (1995) Anal Chem 67:1346
Park J, Paik C-H, Alkire HC (1996) J Electrochem Soc 143(8):L174
Klusmann E, Schultze JW (1997) Electrochim Acta 42:3123
Honda T, Murase K, Hirato T et al (1998) J Appl Electrochem 28:617
Tada E, Sugawara K, Kanekol H (2004) Electrochim Acta 49:1019
Choi Y-S, Shim J-J, Kim J-G (2005) Corrosion 61(5):490
Romankiw LT (1970) IBM Tech Discl Bull 13(1):69
Romankiw LT (1987) Proceedings of the symposium on electrodeposition technology, theory and practice, p 301
Deligianni H, Romankiw LT (1993) IBM J Res Develop 37(2):85
Ji J, Cooper WC, Dreisinger DB et al (1995) J Appl Electrochem 25:642
Deslouis C, Frateur I, Maurin G et al (1997) J Appl Electrochem 27:482
Diaz SL, Mattos OR, Barcia OE, Fabri Miranda J et al (2002) Electrochim Acta 47:4091
Ordine AP, Diaz SL, Margarit ICP et al (2006) Electrochim Acta 51:1480
Nobial M, Devos O, Mattos OR et al (2007) J Electroanal Chem 600:87
Nešić S, Postlethwaite J, Olsen S (1996) Corrosion 54(4):280
George K, Nešić S (2007) Corrosion 63(2):178
Crolet JL, Bonis MR (1983) Corrosion 39(2):39
Acknowledgements
The authors acknowledge Dan Cain, lab technician, for his assistance in fabrication of the surface pH probe. Nicolas Jauseau, and Jin Huang, both PhD students from ICMT, are acknowledged for their help on figure preparation. Jiabin Han expresses his appreciation to postdoctoral researcher Xiu Jiang and PhD student Hui Li for their help in recording the data. The authors also appreciate the financial support from the Corrosion Center Joint Industry Project advisory board members, namely Baker Petrolite, BG Group, BP, Champion Technologies, Chevron, Clariant, Columbia Gas Transmission, ConocoPhillips, Encana, Eni, ExxonMobil, INPEX, IONIK Consulting, MI Production Chemicals, Nalco, Occidental Oil Company, Petronas, Petrobras, PTTEP, Saudi Aramco, Shell, Tenaris and Total.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, J., Brown, B.N., Young, D. et al. Mesh-capped probe design for direct pH measurements at an actively corroding metal surface. J Appl Electrochem 40, 683–690 (2010). https://doi.org/10.1007/s10800-009-0043-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-0043-8