Abstract
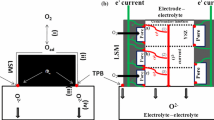
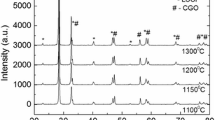
This paper describes the first part of an experimental and theoretical study performed on composite Lanthanum Strontium Manganite (LSM) and Yttria-stabilized Zirconia (YSZ) electrodes. Cathode electrocatalytic activity was investigated using different cell configurations and carrying out potentiodynamic polarisation and electrochemical impedance spectroscopy measurements (EIS). Measurements were carried out at different oxygen partial pressures, overpotentials, temperatures and electrode geometries. In order to identify the main steps involved in cathodic oxygen reduction, the NLLS-Fit procedure was used. The results for different cell geometries agree with each other, suggesting a transition in the overall reaction mechanism, from charge transfer to mass transfer control, at a critical temperature of about 750 °C. The experimental results also show a remarkable effect of electrode thickness on the overall reaction rate, throughout the temperature range tested. A grey level gradient along the thickness of the thicker electrodes were detected by analyzing microscopic images of the cells. These results, together with electrochemical measurements on cathodes with different thickness, confirm that morphology plays a key role in determining the performance of Solid Oxide Fuel Cells (SOFC) composite cathodes.











Similar content being viewed by others
References
Tao S, Irvine JTS, Kilner JA (2005) Adv Mater 17:1734–1737
Peña-Martínez J, Marrero-López D, Pérez-Coll D, Ruiz-Morales JC, Núñez P (2007) Electrochim Acta 52:2950–2958
Shao Z, Haile SM (2004) Nature 431:170–173
Lv H, Tu H-y, Zhao B-y, Wu Y-j, Hu K-a (2007) Solid State Ionics 177:3467–3472
Tao S, Irvine JTS (2003) Nat Mater 2:320–323
Goodenough JB, Huang Y-H (2007) J Power Sources 173:1–10
Jung HY, Kim W-S, Choi S-H, Kim H-C, Kim J, Lee H-W, Lee J-H (2006) J Power Sources 155:145–151
Piccardo P, Chevalier S, Molins R, Viviani M, Caboche G, Barbucci A, Sennour M, Amendola R (2006) Surf Coat Technol 201(7):4471–4475
Ucida H, Arisaka S, Watanabe M (2000) Solid State Ionics 135:347–351
Liu M, Dong D, Peng R, Gao J, Diwu J, Liu X, Meng G (2008) J Power Sources 180:215–220
Schneider LCR, Martin CL, Bultel Y, Dessemond L, Bouvard D (2007) Electrochim Acta 52:3190–3198
Kim J-S, Pyun S-I, Shin H-C, Kang S-JL (2008) J Electrochem Soc 155(7):B762–B769
Hammouche A, Schouler EJL, Henault M (1988) Solid State Ionics 28–30:1205–1207
Mizusaki J, Tagawa H, Naraya K, Sasamoto T (1991) Solid State Ionics 49:111–118
Takeda Y, Sakaki Y, Ichikawa T, Imanishi N, Yamamoto O, Mori M, Mori N, Abe T (1994) Solid State Ionics 72(2):257–264
Barbucci A, Carpanese MP, Reverberi AP, Cerisola G, Blanes M, Cabot PL, Viviani M, Bertei A, Nicolella C (2008) J Appl Electrochem 38:939–945
Nicolella C, Bertei A, Barbucci A, Vatistas N (2008) J Appl Electrochem. doi:10.1007/s10800-008-9691-3
Winkler J, Hendriksen PV, Bonanos N, Mogensen M (1998) J Electrochem Soc 145(4):1184–1192
Adler SB (2002) J Electrochem Soc 149(5):E166–E172
Macdonald JR (1987) Impedance spectroscopy. Wiley, New York
Hsieh G, Mason TO, Garboczi EJ, Pederson LR (1997) Solid State Ionics 96:153–172
Rutman J, Riess I (2008) Placement of reference electrode in solid state electrolyte cells. Solid State Ionics. doi:10.1016/j.ssi.2008.01.071(in press)
Boukamp BA (1986) Solid State Ionics 20:31–44
Fleig J (2002) Solid State Ionics 150:181–193
Stoynov Z, Vladikova D (2005) Differential impedance analysis. Marin Drinov Academic Publishing House, Sofia
Virkar AV, Chen J, Tanner CW, Kim J-W (2000) Solid State Ionics 131:189–198
Kenjo T, Nishiya M (1992) Solid State Ionics 57:295–302
Juhl M, Primdahl S, Manon C, Mogensen M (1996) J Power Sources 61:173–181
Acknowledgements
The authors gratefully acknowledge the financial support of the Italian project “FISR: Nanosistemi Inorganici ed Ibridi per lo Sviluppo e l’Innovazione di Celle a Combustibile”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbucci, A., Paola Carpanese, M., Viviani, M. et al. Morphology and electrochemical activity of SOFC composite cathodes: I. experimental analysis. J Appl Electrochem 39, 513–521 (2009). https://doi.org/10.1007/s10800-008-9708-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9708-y