Abstract
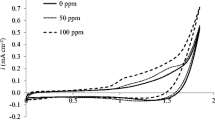
Electrochemical decolourisation of Reactive Orange 16 was carried out in an electrochemical flow-cell, using as working electrodes a Pt thin film deposited on a Ti substrate (Pt/Ti) prepared by the Pechini method and a pure platinum (Pt) foil. Using the Pt/Ti electrodes better results for dye decolourisation were obtained under milder conditions than those used for pure Pt. For the Pt electrode, colour removal of 93 % (λ = 493 nm) was obtained after 60 min, at 2.2 V vs. RHE, using 0.017 mol L−1 NaCl + 0.5 mol L−1 H2SO4 solution. For the Pt/Ti electrode there was better colour removal, 98%, than for the Pt electrode. Moreover, we used 0.017 mol L−1 NaCl solution and the applied potential was 1.8 V. Under this condition after 15 min of electrolysis, more than 80% of colour was removed. The rate reaction constant, assuming a first order reaction, was 0.024 min−1 and 0.069 min−1, for Pt and Pt/Ti electrodes, respectively.





Similar content being viewed by others
References
Kunz A, Peralta-Zamora P, de Moraes SG, Duran N (2002) Quim Nova 25(1):78
Carneiro PA, Osugi ME, Fugivara CS, Boralle N, Furlan M, Zanoni MVB (2005) Chemosphere 59(3):431
Meric S, Kaptan D, Olmez T (2004) Chemosphere 54(3):435
Isik M (2004) Enzyme Microb Technol 35(5):399
Libra JA, Borchert M, Vigelahn L, Storm T (2004) Chemosphere 56(2):167
Ong SA, Toorisaka E, Hirata M, Hano T (2005) Sep Purif Technol 42(3):297
Gutowska A, Kaluzna-Czaplinska J, Jozwiak WK (2007) Dyes Pigm 74(1):41
Bilgi S, Demir C (2005) Dyes Pigm 66(1):69
Baldrian P, Merhautova V, Gabriel J, Nerud F, Stopka P, Hruby M, Benes MJ (2006) Appl Catal B 66(3–4):258
Bredereck K, Schumacher C (1993) Dyes Pigm 21(1):45
Kusic H, Koprivanac N, Srsan L (2006) J Photochem Photobiol A 181(2–3):195
Arslan-Alaton W, Eremektar G, Babuna FG, Selcuk H, Orhon D (2004) Fresenius Environ Bull 13(10):1040
Kraft A, Stadelmann M, Blaschke M (2003) J Hazard Mater 103(3):247
Troster I, Fryda M, Herrmann D, Schafer L, Hanni W, Perret A, Blaschke M, Kraft A, Stadelmann M (2002) Diamond Relat Mater 11(3–6):640
Freitas RG, Oliveira RTS, Santos MC, Bulhoes LOS, Pereira EC (2006) Mater Lett 60(15):1906
Terezo AJ, Pereira EC (2000) Electrochim Acta 45(25–26):4351
Terezo AJ, Pereira EC (2002) Mater Lett 53(4–5):339
Villullas HM, Mattos-Costa FI, Bulhoes LOS (2003) J Electroanal Chem 545:89
Villullas HM, Mattos-Costa FI, Nascente PAP, Bulhoes LOS (2004) Electrochim Acta 49(22–23):3909
Freitas RG, Santos MC, Oliveira RTS, Bulhoes LOS, Pereira EC (2006) J Power Sourc 158(1):164
Freitas RG, Marchesi LF, Oliveira RTS, Mattos-Costa FI, Pereira EC, Bulhoes LOS, Santos MC (2007) J Power Sourc 171(2):373
Reiner A, Steiger B, Scherer GG, Wokaun A (2006) J Power Sourc 156(1):28
Malpass GRP, Motheo AJ (2001) J Appl Electrochem 31(12):1351
Malpass GRP, Miwa DW, Mortari DA, Machado SAS, Motheo AJ (2007) Wat Res 41(13):2969
Feng YG, Smith DW, Bolton JR (2007) J Environ Eng Sci 6(3):277
Catanho M, Malpass GRP, Motheo AJ (2006) Quim Nova 29(5):983
Comninellis C (1994) Electrochim Acta 39(11–12):1857
Bejankiwar R, Lalman JA, Seth R, Biswas N (2005) Water Res 39(19):4715
Acknowledgements
The authors gratefully acknowledge CNPq (grant number: 141464/2005-4) and FAPESP (grant number: 04/09588-1, 03/09933-8 and 05/04708-1)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomes, L., Freitas, R.G., Malpass, G.R.P. et al. Pt film electrodes prepared by the Pechini method for electrochemical decolourisation of Reactive Orange 16. J Appl Electrochem 39, 117–121 (2009). https://doi.org/10.1007/s10800-008-9649-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9649-5