Abstract
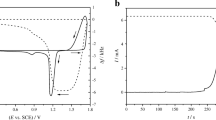
An electrochemical Quartz Crystal Microbalance (EQCM) was used to characterize a mixed metal oxide electrocatalyst, Ir0.15Sn0.85O2, for the Oxygen Evolution Reaction (OER) in acidic medium in the potential range of the pseudo-capacitance reaction, from 0.4 to 1.2 V versus Reversible Hydrogen Electrode (RHE). The EQCM gold tip was characterized in H2SO4 0.05 M and HClO4 0.1 M. Subsequently, Ir0.15Sn0.85O2 powder, synthesized by a sol–gel route, was supported on the tip gold surface for investigations in the same media. The simultaneous measurements of mass variation and current density as functions of potential led to the identification of the chemical species involved in the mass transfer between the oxide and the acidic solution during the pseudo-capacitance reaction.






Similar content being viewed by others
References
Wuebbles DJ, Atul KJ (2001) Fuel Process Technol 71:99
Chalk SG, Miller JF (2006) J Power Sources 159:73
Ecsedy CJ, Murphy CG (1992) Water Environ Res 64:647
Alejaldre C, De Marco F, Finzi U et al (2005) Nucl Fusion 45:A1
Schultz MG, Diehl T, Brasseur GP, Zittel W (2003) Science 302:624
Dunn S (2002) Int J Hydrogen Energy 27:235
Turner JA (2004) Science 305:972
Stavy M (2005) J Sol Energy Eng 127:161
Hetland J, Mulder G (2007) Int J Hydrogen Energy 32:736
Granovskii M, Dincer I, Rosen MA (2007) Int J Hydrogen Energy 32:927
Schug CA (1998) Int J Hydrogen Energy 23:1113
Bilgen E (2004) Sol Energy 77:47
Ogden JM, Williams RH (1990) Int J Hydrogen Energy 15:155
Trasatti S (2001) Port Electrochim Acta 19:197
Rasten E, Hagen G, Tunold R (2003) Electrochim Acta 48:3945
Slavcheva E, Radev I, Bliznakov S et al (2007) Electrochim Acta 52:3889
De Pauli CP, Trasatti S (2002) J Electroanal Chem 538–539:145
Krstajic N, Trasatti S (1998) J Appl Electrochem 28:1291
Cardarelli F, Taxil P, Savall A et al (1998) J Appl Electrochem 28:245
Horvath E, Kristof J, Frost RL et al (2004) J Thermal Anal Calorim 78:687
Chen X, Chen G, Yue PL (2001) J Phys Chem B 105:4623
Comninellis Ch, Vercesi GP (1991) J Appl Electrochem 21:335
De Pauli CP, Trasatti S (1995) J Electroanal Chem 396:161
Lassali TAF, Boots JFC, Bulhoes LOS (1999) Electrochim Acta 44:4203
da Silva LA, Alves VA, de Castro SC, Boots JFC (2000) Colloid Surface A 170:119
Chen X, Chen G, Yue PL (2002) J Phys Chem B 106:4364
Ortiz PI, De Pauli CP, Trasatti S (2004) J New Mat Electrochem Syst 7:153
Chen X, Chen G (2005) J Electrochem Soc 152:J59
Marshall A, Børresen B, Hagen G et al (2007) Energy 32:431
Ardizzone S, Cappelletti G, Ionita M et al (2005) Electrochim Acta 50:4419
Ionita M, Cappelletti G, Minguzzi A et al (2006) J Nanopart Res 8:653
Minguzzi A (2004) Thesis, The University of Milan, Italy
Borgese L (2005) Thesis, The University of Milan, Italy
Ardizzone S, Bianchi CL, Cappelletti G et al (2006) J Electroanal Chem 589:160
Hu C-C, Chang K-H (2000) Electrochim Acta 45:2685
Sauerbrey G (1959) Z Phys 155:206
Vatankhah G, Lessard J, Jerkiewicz G et al (2003) Electrochim Acta 48:1613
Zolfaghari A, Conway BE, Jerkiewicz G (2002) Electrochim Acta 47:1173
Marshall A, Børresen B, Hagen G et al (2006) Electrochim Acta 51:3161
Tian M, Pell WG, Conway BE (2003) Electrochim Acta 48:2675
Watanabe M, Uchida H, Ikeda N (1995) J Electroanal Chem 380:255
Rodriguez Nieto FJ, Andreasen G, Martins ME et al (2003) J Phys Chem B 107:11452
Ardizzone S, Carugati A, Trasatti S (1981) J Electroanal Chem 126:287
Acknowledgements
The financial support by the Oronzio e Niccolò De Nora Foundation, MUR-The University of Milan (FIRST funds) and Fondazione Monte dei Paschi di Siena are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vertova, A., Borgese, L., Cappelletti, G. et al. New electrocatalytic materials based on mixed metal oxides: electrochemical quartz crystal microbalance characterization. J Appl Electrochem 38, 973–978 (2008). https://doi.org/10.1007/s10800-008-9510-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9510-x