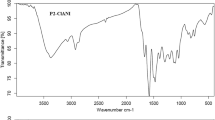
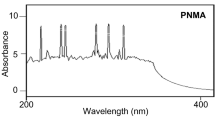
Abstract
The aim of this work was to obtain polyaniline (Pani) and poly(ortho-methoxyaniline) (Poma) by chemical synthesis and to evaluate their corrosion protection properties on carbon steel (CS) and copper (Cu) in an aggressive media such as sodium chloride. The syntheses of the polymers were carried out by chemical oxidation of the monomers by (NH4)2S2O8 in nitric acid solutions. Under these conditions, the polymers were obtained in the oxidized form, dissolved in 1-methyl-2-pyrrolidone and casted by solvent evaporation onto the metallic substrates (carbon steel and copper) for corrosion evaluation. The morphology of the polymers was evaluated by scanning electron microscopy (SEM) and atomic force microscopy (AFM). The electrochemical behavior was determined by open circuit potential (OCP) measurements and polarization curves (PC). The best results were obtained with Pani because this polymer film participates in the formation of an oxide film at the polymer–metal interface, a phenomenon which is not observed with Poma. This oxide film increases the barrier effect that the polymeric film has by itself.







Similar content being viewed by others
References
Graedel TE, Frankenthal RP (1990) J Electrochem Soc 137:2385
Feliu S, Morcillo M, Feliu S Jr (1993) Corr Sci 34:403
Morcillo M, Almeida E, Rosales B, Uruchurtu J, Marrocos M (1998) Corrosión y Protección de Metales en las Atmósferas de Iberoamérica, CYTED, Madrid, España
Johansson E, Leygraf C, Rendahl B (1998) Brit Corr J 33:59
Odnevall Wallinder I, Verbiest P, He W, Leygraf C (2000) Corr Sci 42:1471
Jeffrey R, Melchers RE (2002) Brit Corr J 37:99
Corvo F, Mendoza AR, Autie M, Betancourt N (1997) Corr Sci 39:815
Nekrasov A, Ivanov V, Vannikov A (2001) Electrochim Acta 46:3301
Bernard M, Joiret S, Hugot-Le Goff A, Viet Phong P (2001) J Electrochem Soc 148:B12
Widera J, Skompska M, Jackowska K (2001) Electrochim Acta 46:4125
Lu JL, Liu NJ, Wang XH, Li J, Jing XB, Wang FS (2003) Synth Met 135–136:237
Vera R, Romero H, Ahumada E (2003) J Chil Chem Soc 48:35
Spinks GM, Dominis AJ, Wallace GG (2003) Corrosion 59:22
Spinks GM, Dominis AJ, Wallace GG, Tallman DE (2002) J Solid State Electrochem 6:85
Kinlen PJ, Silverman DC, Jeffreys CR (1997) Synth Met 85:1327
Tallman DE, Spinks G, Dominis A, Wallace G (2002) J Solid State Electrochem 6:73
Camalet JL, Lacroix JC, Aeiyach S, Lacaze PC (1998) J Electroanal Chem 445:124
Wessling B (2003) Synth Met 135–136:265
Fahlman M, Jasty S, Epstein AJ (1997) Synth Met 85:1323
Epstein AJ, Smallfield J, Guan H, Fahlman M (1999) Synth Met 102:1374
Meneguzzi A, Ferreira CA, Pham MC, Delamar M, Lacaze PC (1999) Electrochim Acta 44:2149
Gasparac R, Martin C (2001) J Electrochem Soc 148:138
Choi S, Park S (2002) J Electrochem Soc 149:E26
Tallman DE, Pae Y, Blerwagen GP (1999) Corrosion, 55:779
Santos JR, Mattoso LH, Motheo AJ (1998) Electrochim Acta 43:309
Brusic V, Angelopoulus M, Graham T (1997) J Electrochem Soc 144:436
Fries J, Getrost H, (1977) in: Darmstadt M (ed) Organic reagents for trace analysis, pp 141–143
Acknowledgements
The authors thank the Dirección de Investigación de la Pontificia Universidad Católica de Valparaíso, Chile, for financial support through Project No 125.763.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vera, R., Schrebler, R., Cury, P. et al. Corrosion protection of carbon steel and copper by polyaniline and poly(ortho-methoxyaniline) films in sodium chloride medium. Electrochemical and morphological study. J Appl Electrochem 37, 519–525 (2007). https://doi.org/10.1007/s10800-006-9284-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-006-9284-y