Abstract
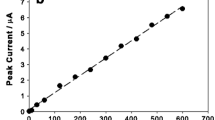
A simply prepared carbon nano tube paste electrode (CNTPE) was utilized for monitoring mercury ion concentration using the cyclic voltammetry (CV) method and the square wave anodic stripping voltammetric (SWASV) method. The CNTPE was compared with various conventional electrodes. The CNTPE method was applied to determine the concentration of trace levels of Hg(II) in several water samples, which yielded a relative error of 0.6% with a concentration of 0.20 mg L−1 Hg(II). It was deposited at −0.5 V (vs Ag/AgCl), which was subsequently reduced to +0.20 V to strip it on the CNTPE. The optimal experimental conditions for the analysis were found to be as follows: pH value of 4 for the medium; deposition potential of −0.5 V; deposition time of 210 s; SW frequency of 40 Hz; SW amplitude of 100 mV, and step potential of 25 mV. Given these optimum conditions, a linear range was observed within the concentrations of 1.0–25.0 μg L−1 and 40.0–200.0 μg L−1. The detection limit was found to be 0.42 μg L−1.
Similar content being viewed by others
References
M. Horvat J. Kotnik M. Logar V. Fajon T. Zvonari N. Pirrone (2003) Atmos. Environ. 1 S93 Occurrence Handle10.1016/S1352-2310(03)00249-8
C.F. Harrington S.A. Merson T.M. D’ Silva (2004) Anal. Chim. Acta 505 247 Occurrence Handle10.1016/j.aca.2003.10.046 Occurrence Handle1:CAS:528:DC%2BD2cXhtVKnsrk%3D
J.D. Laumb S.A. Benson E.A. Olson (2004) Fuel Process. Technol. 85 577 Occurrence Handle1:CAS:528:DC%2BD2cXjtFCqsbw%3D
E.M. Sunderland G.L. Chmura (2000) Sci. Total Environ. 256 39 Occurrence Handle1:CAS:528:DC%2BD3cXjs1WjurY%3D Occurrence Handle10898386
L.D. Lacerda R.V. Marins (1997) J. Geochem. Explor. 58 223 Occurrence Handle1:CAS:528:DyaK2sXjvVWjur0%3D
R.D. Riso M. Waeles P. Monbet C.J. Chaumery (2000) Anal. Chim. Acta 410 97 Occurrence Handle1:CAS:528:DC%2BD3cXitVSmu7c%3D
S. Sholupov S. Pogarev V. Ryzhov N. Mashyanov A. Stroganov (2004) Fuel Process. Technol. 85 473 Occurrence Handle1:CAS:528:DC%2BD2cXjtFCqs7s%3D
L.M. Dong X.P. Yan Yan Li Y. Jiang S.W. Wang D.Q. Jiang (2004) J. Chromatogr. 1036 119 Occurrence Handle1:CAS:528:DC%2BD2cXjtFantbo%3D
L.N. Liang G.B. Jiang J.F. Liu J.T. Hu (2003) Anal. Chim. Acta 477 131 Occurrence Handle1:CAS:528:DC%2BD38Xpslais70%3D
G. Centineo E.B. Gonzalez A.S. Medel (2004) J. Chromatogr. 1034 191 Occurrence Handle1:CAS:528:DC%2BD2cXisVGhtrs%3D
H. Matusiewicz R.E. Sturgeon (1996) Spectrochim. Acta 51 377
W. Wiyaratn M. Somasundrum W. Surareungchai (2004) Anal. Chem. 76 859 Occurrence Handle1:CAS:528:DC%2BD3sXhtVSiur%2FP Occurrence Handle14750886
T.H. Lu J.F. Huang I.W. Sun (2001) Anal. Chim. Acta 21701 1
M. Paneli, H. Ouguenoune, F. David, A. Bolyos Anal. Chim. Acta (1995) 177.
J. Wang J. Lu (2000) Electrochem. Commun. 2 390 Occurrence Handle1:CAS:528:DC%2BD3cXjvFWqtrc%3D
M.H. Pournaghi-Azar M.R. Ramazani (2002) Electroanalysis. 14 1203 Occurrence Handle1:CAS:528:DC%2BD38XnvFGitbw%3D
G.B. El-Hefnawey I.S. El-Hallag E.M. Ghoneim M.M. Ghoneim (2004) J. Pharmaceut. Biomed. Anal. 34 75 Occurrence Handle1:CAS:528:DC%2BD2cXltFSrug%3D%3D
S.Y. Ly J.I. Chae Y.S. Jung W.W. Jung H.J. Lee S.H. Lee (2004) Nahrung/Food 48 201 Occurrence Handle1:CAS:528:DC%2BD2cXmtlSnsr8%3D
S.Y. Ly D.H. Kim M.H. Kim (2002) Talanta 58 919 Occurrence Handle1:CAS:528:DC%2BD38XotVyqt78%3D
K.K. Shiu K. Shi (1998) Electroanalysis 10 959 Occurrence Handle1:CAS:528:DyaK1cXnslWmsr0%3D
J.M. Pingarr I. OrtizHernadez A. Gonzaez-Cortes P. Yanez-Sedeno (2001) Anal. Chim. Acta 439 281
M.J. Gonzalezdela Huebra P. Hernadez Y. Ballesteros L. Hernadez (2001) Talanta 54 1077 Occurrence Handle1:CAS:528:DC%2BD3MXkslGjsL8%3D
X. Zhang B. Ogorevc M. Rupnik M. Kreft R. Zorec (1999) Anal. Chim. Acta 378 135 Occurrence Handle1:CAS:528:DyaK1cXntVGjsL4%3D
J. Wang S.B. Hocevar B. Ogorevc (2004) Electrochem. Commun. 6 176 Occurrence Handle1:CAS:528:DC%2BD2cXksFamtQ%3D%3D
S. Lu K. Wu X. Dang S. Hu (2004) Talanta 63 653 Occurrence Handle1:CAS:528:DC%2BD2cXjvVKiu70%3D
J. Wang M. Musameh (2004) Anal. Chim. Acta 511 33 Occurrence Handle1:CAS:528:DC%2BD2cXjvVGhsr8%3D
S. Lu K. Wu X. Dang S. Hu (2004) Talanta 63 653 Occurrence Handle1:CAS:528:DC%2BD2cXjvVKiu70%3D
J. Li S. Liu X. Mao P. Gao Z. Yan (2004) J. Electroanal. Chem. 561 137 Occurrence Handle1:CAS:528:DC%2BD3sXptlCmur4%3D
P.C. Pandey S. Upadhyay B. Upadhyay (1997) Anal. Biochem. 252 136 Occurrence Handle1:CAS:528:DyaK2sXmsVWkt7w%3D Occurrence Handle9324951
30. J. Wang, U.A. Kirgoz, J.W. Mo, J. Lu, A.N. Kawde and A. Muck, Electrochem. Commun. (2001) 203
Acknowledgement
This work was supported by grant No. (R01-2003-000-10530-0) from Ministry of Science & Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
LY, S., Kim, S., Kim, T. et al. Measuring mercury ion concentration with a carbon nano tube paste electrode using the cyclic voltammetry method. J Appl Electrochem 35, 567–571 (2005). https://doi.org/10.1007/s10800-005-2058-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10800-005-2058-0