Abstract
Purpose
This study aimed to evaluate the clinical outcomes up to 10 years after Descemet membrane endothelial keratoplasty (DMEK).
Methods
In this retrospective, consecutive, single-center case series the medical files of eyes which have received DMEK between 2009 and 2012 for the treatment of endothelial dysfunction was evaluated regarding follow-up time and clinical outcomes.
Annual examinations of best-corrected visual acuity (BCVA), endothelial cell density (ECD), central corneal thickness (CCT) of 66 eyes which fulfilled the criterion of a minimum of 8 years follow-up were analyzed.
Results
BCVA improved from 0.55 ± 0.37 logMAR (n = 54) to 0.15 ± 0.11 (n = 47) in eyes without ocular comorbidities one year after DMEK (p < 0.001), and remained stable up to 10 years after DMEK. Mean ECD decreased to 744 ± 207 cells/mm2 (n = 39) after 9 years, and to 729 ± 167 cells/mm2 (n = 21) after 10 years, respectively. CCT decreased from 650 ± 67 μm before DMEK to 525 ± 40 μm (n = 56) after 1 year, increasing slowly to 563 ± 40 µm (n = 39) after 9 years, and to 570 ± 42 µm (n = 21) after 10 years, respectively. Graft failure occurred in 4 of 66 eyes after year 8. These 4 eyes required repeat DMEK after 101–127 months.
Conclusion
This study shows the long-term outcomes in a small subset of DMEK grafts. Visual acuity remained stable in spite of slowly increasing corneal thickness and diminishing endothelial cell density during the 10-year period after DMEK.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Descemet membrane endothelial keratoplasty (DMEK) has become a favorite method to treat endothelial dysfunction. The advantages of the method are rapid visual rehabilitation, near-normal anatomical structure of the cornea, and a low rate of graft rejections. [1,2,3,4,5,6,7,8,9].
The surgical technique encountered resistance by many corneal surgeons in the first years after introduction due to difficulties during the learning curve and the initially non-standardized procedure [10]. An argument against DMEK, which has been expressed in the past, is the challenging preparation of the vulnerable, thin graft with potential high endothelial cell loss during preparation and surgery diminishing survival of the graft. Much effort has been made to lower the rate of complications and thereby increase the reproducibility of the method, for example by reducing the rate of graft detachments. [11,12,13].
By this means, DMEK has gained popularity and has become more widespread in the US and Europe [14, 15]. Fuchs’ endothelial corneal dystrophy is the most common indication for keratoplasty worldwide, and the number of DMEK surgeries has been doubling every year between 2011 and 2014 in the United States, although Descemet’s stripping automated endothelial keratoplasty (DSAEK) is still very popular [14,15,16]. In Germany, DMEK has surpassed DSAEK as most common keratoplasty technique already in 2012. [15].
However, in contrast to the century-long experience gained in penetrating keratoplasty (PK), DMEK is a relatively new technique with few long-term data. The purpose of this study was to evaluate if the success of DMEK shown for the first years after surgery persists beyond the time frame of 5 years using a standardized surgical technique. [17,18,19,20].
Methods
Patients
In this retrospective, single-center cohort study, the long-term results after DMEK were evaluated. The main inclusion criterion was a minimum postoperative follow-up interval of 8 years.
The medical files of all DMEK surgeries performed between July 2009 and June 2012 (n = 450) were analyzed regarding the follow-up time. The follow-up time of all eyes undergoing surgery during this interval was obtained and the graft survival rate was calculated (data shown as supplemental Table 1 and supplemental Fig. 1). 66 eyes fulfilled the inclusion criterion of a follow-up of at least 8 years. Participation in all intermediate follow-up examinations was not required for patients to be included.
The surgeries had been performed between July 2009 and June 2012 at the Department of Ophthalmology, Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Erlangen, Germany. The male/female ratio was 47%/53%, the mean age at time of surgery was 63 ± 9 years. Indication for DMEK was Fuchs endothelial corneal dystrophy in 60 eyes (91%), DMEK after failed DSAEK in 4 eyes (6%), and DMEK after failed DMEK in 2 eyes (3%). Mean follow-up time was 108 ± 11 months. 39/66 eyes (59%) fulfilled a follow-up of 9 years, 21/66 eyes (32%) a follow-up of 10 years.
The Institutional Review Board (IRB)/ Ethics Committee of the Friedrich-Alexander University Erlangen-Nürnberg approved the study (Approval ID: 64_15 Bc). The study was in adherence to the tenets of the Declaration of Helsinki. Informed consent to surgery was obtained from all patients prior to surgery.
Corneal grafts
The donor corneoscleral tissues were obtained from eye banks in the United States (hypothermic storage at 4 °C in Optisol-GS, n = 20, 30%), and from Europe (via German Society for Tissue Transplantation (DGFG); organ-culture at 34 °C in Dulbecco’s modifies Eagle medium, n = 46, 70%). Mean donor age was 69 ± 13 years, mean death-to preservation time was 9 ± 5 h, and mean culture storage duration was 344 ± 172 h.
Surgical technique
All surgeries were performed under general anesthesia by 3 different surgeons. On the day before DMEK, two Nd:YAG-laser iridotomies were performed to prevent postoperative pupillary block.
The surgical technique as described by Kruse et al. was used for graft preparation and transplantation [18,19,20]. Graft preparation was performed on the day of surgery by the surgeons themselves. Standard graft size was 8.0 mm. Triple DMEK defined as DMEK combined with cataract surgery was performed in 28 cases (42%) [1]. We used a spherical single-piece acrylic intraocular lens (46 S AcriSmart; Carl Zeiss Meditec, Jena, Germany) for implantation. [1, 3]
A repeat air injection into the anterior chamber was performed in case of clinically significant graft detachment during the early postoperative period. Graft detachment was considered significant when there was a gap of more than one full corneal thickness over more than one quadrant of the transplant. This so-called rebubbling procedure was necessary in 45% (n = 30) of eyes (one rebubbling: n = 20; two rebubblings: n = 5; three rebubblings: n = 5). The mean interval between DMEK surgery and last rebubbling was 10 ± 7 days (median 7 days, range 3–35 days).
Postoperative medication
The postoperative standard treatment regimen consisted of topical pilocarpine 1% four times a day until the air bubble in the anterior chamber had been resorbed completely, topical ofloxacin 0.3% twice a day for 10 days, hyperosmolar eye drops 5 times a day, and topical prednisolone acetate 1% five times a day. Prednisolone eye drops were tapered monthly over 5 months and continued once a day during the first postoperative year.
Statistics and measurements
The main outcome parameters of this study were best-corrected distance visual acuity (BCVA) in logarithm of the minimum angle of resolution (logMAR) units, endothelial cell density (ECD) in cells/mm2, central corneal thickness (CCT) in µm, and frequency of graft failures after the 8-years follow-up. BCVA was measured by routine visual acuity tests with number of optotypes using optimal spectacle correction.
Follow-up examinations were performed at 1 and 3 months after surgery, then annually up to 10 years after DMEK. Only the annual examinations were analyzed in this study.
CCT values were measured using Scheimpflug imaging (pachymetry at corneal apex, Pentacam; Oculus, Wetzlar, Germany); only scans with approval of high quality by the device were taken into account.
Endothelial cell density was analyzed using specular microscopy by two different manufacturers: from 2009 until September 2016, the device SeaEagle (HAI Laboratories, Lexington, MA, USA) was applied. Tomey specular microscope EM-4000 (Tomey GmbH Technology and vision, Nürnberg, Germany) was used from October 2016 on. The automatic cell border analysis provided by the devices was used for endothelial cell density calculation. All measurements and cell border alignments were checked by an independent examiner and corrected manually in case of misalignment.
In case of low quality of the CCT or ECD measurement, the values were not evaluated in this study. Due to the retrospective design of this study, a repeat measurement was not possible afterward.
The program SPSS for Windows (version 24, SPSS Inc, Chicago, IL, USA) was used for statistical analysis of the data. We compared measurements using Wilcoxon signed-rank test with a significance level set at 5% (P = 0.05). Normal distribution of the data was examined with the Kolmogorov–Smirnov test showing no normal distribution.
Results
Visual acuity
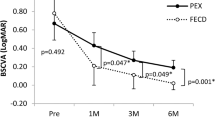
Mean preoperative best-corrected visual acuity (BVCA) ± SD was 0.63 ± 0.43 logMAR improving to 0.19 ± 0.14 after 1 year (P < 0.001). Afterward, visual acuity remained stable up to 10 years after DMEK except for a slight decrease of BCVA after 8 years. BCVA (logMAR) was 0.19 ± 0.21 at year 8 (n = 54), 0.18 ± 0.20 at year 9 (n = 39), and 0.13 ± 0.18 at year 10 (n = 21) (Table 1). A visual acuity of 20/40 or better was achieved in 87% of eyes after 8 years, 85% after 9 years, and 91% after 10 years, respectively. 45% of eyes had a BCVA of 20/25 or better after 8 years, 51% after 9 years, 67% after 10 years, respectively.
Eleven eyes included in this study had ocular comorbidities influencing the visual potential. Ocular comorbidities comprised amblyopia (n = 3), epithelial basement membrane dystrophy with irregular astigmatism (n = 3), optic nerve atrophy (n = 2), macular pucker (n = 1), diabetic retinopathy (n = 1), and repeat hemorrhages into the anterior chamber due to a systemic blood coagulation disorder (n = 1).
Eyes without vision-impairing comorbidities (n = 55) had a preoperative BCVA ± SD (logMAR) of 0.55 ± 0.37 improving to 0.15 ± 0.11 (P < 0.001) one year after DMEK (Table 1). After the first postoperative year, the mean visual acuity values remained stable up to 10 years after DMEK (P > 0.05), except for a slight decrease of BCVA after 8 years. Mean BCVA (logMAR) accounted for 0.13 ± 0.11 (n = 45), 0.12 ± 0.12 (n = 30) and 0.10 ± 0.14 (n = 15) after 8, 9 and 10 years, respectively. In the group of eyes without comorbidities, a visual acuity of 20/40 or better was reached in 98% of eyes 8 years, in 97% 9 years, and in 95% 10 years postoperatively. 53% of eyes had a BCVA of 20/25 or better after 8 years, 61% after 9 years, and 74% after 10 years, respectively.
59% of eyes (n = 39) attended the 9-year visit, 32% (n = 21) the 10-year visit. In order to avoid a bias by comparing the mean values of different numbers of eyes attending the annual visits, we added an analysis of BCVA/ECD/CCT of the same eyes (n = 21) which have completed the 10-year visit (Supplemental Table 2).
Endothelial cell density
Mean donor ECD ± SD was 2582 ± 212 cells/mm2 and decreased to 1504 ± 275 cells/mm2 after the first postoperative year (P < 0.001) (table 2). The cell density remained stable up to five years but decreased considerably afterward: ECD was 739 ± 197 cells/mm2 (n = 45), 744 ± 207 cells/mm2 (n = 39), and 729 ± 167 cells/mm2 (n = 21) at the 8-, 9-, and 10-year visit, respectively. Thereby, endothelial cell loss compared to the baseline measurement accounted for 71, 71, and 72%, after 8, 9, and 10 years, respectively. The decline of ECD between years 5 and 6, and between year 6 and 7 was statistically significant (P < 0.001).
Central corneal thickness
Mean CCT decreased from 650 ± 67 µm at baseline to 525 ± 40 µm at the 1-year follow-up visit (− 19%, P < 0.001). A slight increase of mean CCT to 532 ± 45 µm after 2 years (P = 0.001) was noticed (Table 2). CCT values increased significantly between year 3 and 4 and between 4 and 5, and remained stable at an average of 559 ± 48 µm (n = 54), 563 ± 40 µm (n = 39), and 570 ± 42 µm (n = 21), after 8, 9, and 10 years, respectively. The CCT value before DMEK surgery (CCT at baseline) of eyes in which DMEK had been performed for the indication of failed DSAEK (n = 4) was excluded from the analysis since the cornea thickness is increased in these eyes at the preoperative measurement because of the DSAEK graft.
Complications
Cystoid macular edema was detected in 6 eyes (9%) during the first postoperative year by optical coherence tomography. Immunologic graft rejection or other complications as infectious keratitis, which have been described after DMEK, did not occur in any patient. Steroid-induced postoperative glaucoma occurred in 6 eyes (9%).
Four eyes of the 66 eyes included in this long-term study suffered from a graft failure after the 8-year visit. They underwent repeat DMEK after 101, 104, 114, and 127 months, respectively. There were no signs of graft rejection; all graft failures occurred due to late endothelial failure.
Discussion
Most studies concerning the outcomes of DMEK surgery describe the results in the first 6–24 months postoperatively [3, 5, 21,22,23]. The aim of this study was the evaluation of the 10-year success of DMEK surgery. In our investigation of the 5-year outcomes, we had found stable visual acuity and ECD values, and a graft survival rate of 95% [17]. The 5-year results after DMEK of 500 eyes have been published recently by the Melles group: Visual acuity improved up to 36 months and remained stable afterward [24]. Endothelial cells decreased by approximately 7% per year after the first year and accounted for 55% at 5 years.
Comparative analyses of DMEK with the conventional keratoplasty methods for the treatment of FECD exist also only for the early and intermediate postoperative phase up to five years [3, 25, 26]. Woo et al. compared the 5-year outcomes of DMEK, DSAEK, and PK for the indication FECD and pseudophakic bullous keratopathy [26]. Graft survival was best in the DMEK group (97.4%), even though the endothelial cell loss after 1 year was highest in the DMEK group (39.9%). Price et al. did not find a significant difference in graft survival and ECD after DMEK compared to DSAEK at 5 years. [27] Up to now, there is no long-term (10 years) data comparing the outcomes of DMEK with DSAEK or PK.
In the analysis of the so-called midterm results, the outcomes of 250 eyes which underwent DMEK with a follow-up time up to 7 years were analyzed by Ham et al. [28] The cumulative graft survival rate was excellent (96%) and visual acuity remained stable up to 4–7 years. ECD decreased slowly with an annual decline by 9% after the first 6 months.
The longest cohort study after DMEK has been published by the Melles group recently [29]. They described a similar endothelial cell loss (− 68%) in 57 eyes out of the first 100 DMEK patients, excellent BCVA results, and a graft survival probability of 0.79 at ten years.
The highest loss of endothelial cells occurs perioperatively, most likely attributable to the graft preparation and the transplantation procedure itself. After this early cell loss, which amounts to 42% after the first postoperative month, the ECD decreased by further 30% compared to baseline in the following 9–10 years in the present study [17]. The observed cell loss of 29% between the 1st and 10th year after surgery would represent an annual cell loss of 3%. If we use this data to extrapolate further endothelial cell loss, the critical borderline for corneal compensation, which is usually assigned at values about 500 cells/mm2, might be crossed 12–13 years after DMEK leading to endothelial decompensation and graft failure [30]. However, Baydoun et al. have shown that 5% of corneas which were clear 7 years after DMEK had an ECD of less than 500 cells/mm2. [31] Therefore, extrapolation of endothelial cell loss and prediction of graft failure remains difficult.
Interestingly, the endothelial cell loss at 10 years we detected after DMEK (72%) is comparable to the 10-year data obtained for PK (76%) observed in the Cornea Donor Study [32]. The authors of the Cornea Donor Study reported that some corneas remained clear although the ECD dropped below 500 cells/mm2.
Recently, several in vitro and in vivo studies provided evidence for the role of the aqueous humor on endothelial cell survival: Total antioxidant capacity and ascorbic acid levels are decreased in eyes with lower endothelial cell densities [33]. Furthermore, several cytokines, for example, interleukin-6, are elevated preoperatively in the aqueous humor in eyes with subsequent graft failure after keratoplasty [34, 35]. In FECD eyes, an upregulation of several genes (e.g. N-cadherin, alpha-SMA, etc.) has been shown in endothelial cells adjacent to guttae leading to an altered microenvironment on endothelial level [36]. These findings might be an interesting therapeutical approach to influence endothelial cell survival.
The loss of endothelial cells we found in our first cohort study about the 5-year results after DMEK was stable during the 5-year period after an initial relatively high loss by 42% at the 1-year visit. The current study, which comprises the data of a subgroup of the patients of the first study with a longer follow-up, showed a further decline after the first five years with a distinct decrease after 6–7 years. This might be attributed to the change in our endothelial cell measurement devices in 2016, which is a major shortage of this study limiting the validity of our ECD results. Unfortunately, internal comparison and validation of the devices are not possible since the older one is not available anymore. To our knowledge, there exist no studies comparing the variability of measurements taken by these two devices.
Another major limitation of this study is the relatively small number of patients fulfilling the required follow-up time of 8–10 years.
The retrospective setting of the study contributed to the high drop-out rate of the study. Patients undergoing DMEK at our Department visit the out-patient clinic after 1 month, 3 months, and annually after the first year. A reason for the high loss of patients for the long-term controls is attributed to the fact, that in the years between 2009 and 2011 only few sites in Europe offered the innovation of DMEK surgery. Therefore, most patients traveled a long distance for the surgery and were not willing or able to attend the annual follow-up visits at our clinic. The mean age of patients at time of surgery was 63 ± 9 years, i.e. the average age was 73 years at the 10-year follow-up, making annual visits more troublesome.
Another limitation of this study is the lack of eyes with pseudophakic bullous keratopathy (PBK). This is caused mainly by the small number of patients included and by the lower rate of PBK compared to FECD in eyes undergoing endothelial keratoplasty in our department. Further studies addressing the long-term outcome of DMEK in PBK are necessary.
Since the surgical technique has been standardized by several modifications, complications—especially graft detachments—have become less frequent in the last years [38]. All eyes included in this study underwent surgery in the early period of DMEK evolution. Thereby, the relatively rebubbling rate in this study cohort can be explained. One might expect a superior graft survival and long-term stability of DMEK in the future due to the standardized surgical technique. However, in two comparative studies of eyes in which DMEK surgery was performed in different stages of the learning curve of corneal surgeons, no significant difference of the ECD loss between less and more experienced surgeons was found during the follow-up period of 6 and 12 months, respectively, even though the rate of graft detachments dropped dramatically during the study period because of further technical improvements. [37, 38].
Further studies about the long-term course after DMEK are desired since it is unknown if DMEK grafts are going to fail after 10–15 years when the endothelial cell count crosses the border of corneal compensatory capability. In addition, the long-term prognosis of eyes with difficult preoperative situations (for example prior glaucoma surgery) or in eyes with indications other than FECD should be analyzed in the future.
The results of this evaluation of the 10-year outcomes in a small subset of DMEK grafts show the natural development of these eyes with stable visual function over 10 years, despite a clear trend of endothelial cell loss over time. The value of this study is curtailed by the high loss to follow-up in the original DMEK cohort. Prospective longitudinal cohort studies are needed to determine the long-term survival of DMEK grafts.
Availability of data and material
All authors state that data and materials comply with field standards.
Abbreviations
- BCVA:
-
Best corrected visual acuity
- CCT:
-
Central corneal thickness
- DMEK:
-
Descemet membrane endothelial keratoplasty
- DSAEK:
-
Descemet’s stripping automated endothelial keratoplasty
- ECD:
-
Endothelial cell density
- FECD:
-
Fuchs’ endothelial corneal dystrophy
- logMAR:
-
Logarithm of the minimum angle of resolution
- PK:
-
Penetrating keratoplasty
- SD:
-
Standard deviation
References
Laaser K, Bachmann BO, Horn FK, Cursiefen C, Kruse FE (2012) Descemet membrane endothelial keratoplasty combined with phacoemulsification and intraocular lens implantation: advanced triple procedure. Am J Ophthalmol 154(1):47-55.e2. https://doi.org/10.1016/j.ajo.2012.01.020
Livny E, Parker JS, van der Kaaij M, Haasdijk ED, van der Wees J, Bruinsma M, Melles GR (2014) Postmortem ultrastructural analysis of a cornea transplanted with Descemet membrane endothelial keratoplasty. Cornea 33(8):790–794. https://doi.org/10.1097/ICO.0000000000000156
Tourtas T, Laaser K, Bachmann BO, Cursiefen C, Kruse FE (2012) Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Am J Ophthalmol 153(6):1082–90.e2. https://doi.org/10.1016/j.ajo.2011.12.012
Goldich Y, Showail M, Avni-Zauberman N, Perez M, Ulate R, Elbaz U, Rootman DS (2015) Contralateral eye comparison of descemet membrane endothelial keratoplasty and descemet stripping automated endothelial keratoplasty. Am J Ophthalmol 159(1):155–9.e1. https://doi.org/10.1016/j.ajo.2014.10.009
Price MO, Giebel AW, Fairchild KM, Price FW Jr (2009) Descemet’s membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology 116(12):2361–2368. https://doi.org/10.1016/j.ophtha.2009.07.010
Anshu A, Price MO, Price FW Jr (2012) Risk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplasty. Ophthalmology 119(3):536–540. https://doi.org/10.1016/j.ophtha.2011.09.019
Price MO, Price FW Jr, Kruse FE, Bachmann BO, Tourtas T (2014) Randomized comparison of topical prednisolone acetate 1% versus fluorometholone 0.1% in the first year after descemet membrane endothelial keratoplasty. Cornea, 33(9), 880–886. https://doi.org/10.1097/ICO.0000000000000206
Ćirković A, Schlötzer-Schrehardt U, Weller JM, Kruse FE, Tourtas T (2015) Clinical and ultrastructural characteristics of graft failure in DMEK: 1-year results after repeat DMEK. Cornea 34(1):11–17. https://doi.org/10.1097/ICO.0000000000000295
Weller JM, Tourtas T, Kruse FE, Schlötzer-Schrehardt U, Fuchsluger T, Bachmann BO (2015) Descemet membrane endothelial keratoplasty as treatment for graft failure after descemet stripping automated endothelial keratoplasty. Am J Ophthalmol 159(6):1050-1057.e2. https://doi.org/10.1016/j.ajo.2015.03.010
Dapena I, Ham L, Droutsas K, van Dijk K, Moutsouris K, Melles GR (2011) Learning curve in Descemet’s membrane endothelial keratoplasty: first series of 135 consecutive cases. Ophthalmology 118(11):2147–2154. https://doi.org/10.1016/j.ophtha.2011.03.037
Ćirković A, Beck C, Weller JM, Kruse FE, Tourtas T (2016) Anterior chamber air bubble to achieve graft attachment after DMEK: Is bigger always better? Cornea 35(4):482–485. https://doi.org/10.1097/ICO.0000000000000753
Tourtas T, Schlomberg J, Wessel JM, Bachmann BO, Schlötzer-Schrehardt U, Kruse FE (2014) Graft adhesion in descemet membrane endothelial keratoplasty dependent on size of removal of host’s Descemet membrane. JAMA Ophthalmol 132(2):155–161. https://doi.org/10.1001/jamaophthalmol.2013.6222
von Marchtaler PV, Weller JM, Kruse FE, Tourtas T (2018) Air versus sulfur hexafluoride gas Tamponade in Descemet membrane endothelial keratoplasty: a fellow eye comparison. Cornea 37(1):15–19. https://doi.org/10.1097/ICO.0000000000001413
Park CY, Lee JK, Gore PK, Lim CY, Chuck RS (2015) Keratoplasty in the United States: a 10-Year Review from 2005 through 2014. Ophthalmology 122(12):2432–2442. https://doi.org/10.1016/j.ophtha.2015.08.017
Flockerzi E, Maier P, Böhringer D, Reinshagen H, Kruse F, Cursiefen C, Reinhard T, Geerling G, Torun N, Seitz B, all German Keratoplasty Registry Contributors (2018). Trends in corneal transplantation from 2001 to 2016 in Germany: a report of the DOG-section cornea and its keratoplasty registry. Am J Ophthalmol 188, 91–98. https://doi.org/10.1016/j.ajo.2018.01.018
Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, Thuret G (2016) Global survey of corneal transplantation and eye banking. JAMA ophthalmology 134(2):167–173. https://doi.org/10.1001/jamaophthalmol.2015.4776
Schlögl A, Tourtas T, Kruse FE, Weller JM (2016) Long-term clinical outcome after descemet membrane endothelial keratoplasty. Am J Ophthalmol 169:218–226. https://doi.org/10.1016/j.ajo.2016.07.002
Kruse FE, Schrehardt US, Tourtas T (2014) Optimizing outcomes with Descemet’s membrane endothelial keratoplasty. Curr Opin Ophthalmol 25(4):325–334. https://doi.org/10.1097/ICU.0000000000000072
Kruse FE, Laaser K, Cursiefen C, Heindl LM, Schlötzer-Schrehardt U, Riss S, Bachmann BO (2011) A stepwise approach to donor preparation and insertion increases safety and outcome of Descemet membrane endothelial keratoplasty. Cornea 30(5):580–587. https://doi.org/10.1097/ico.0b013e3182000e2e
Bachmann BO, Laaser K, Cursiefen C, Kruse FE (2010) A method to confirm correct orientation of descemet membrane during descemet membrane endothelial keratoplasty. Am J Ophthalmol 149(6):922-925.e2. https://doi.org/10.1016/j.ajo.2010.01.005
Guerra FP, Anshu A, Price MO, Giebel AW, Price FW (2011) Descemet’s membrane endothelial keratoplasty: prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology 118(12):2368–2373. https://doi.org/10.1016/j.ophtha.2011.06.002
Monnereau C, Quilendrino R, Dapena I, Liarakos VS, Alfonso J.F, Arnalich-Montiel F, Böhnke M, Pereira NC, Dirisamer M, Parker J, Droutsas K, Geerling G, Gerten G, Hashemi H, Kobayashi A, Naveiras M, Oganesyan O, Orduña Domingo E, Priglinger S, Stodulka P, … Melles GR (2014). Multicenter study of descemet membrane endothelial keratoplasty: first case series of 18 surgeons. JAMA Ophthalmol 132(10), 1192–1198. https://doi.org/10.1001/jamaophthalmol.2014.1710
Rodríguez-Calvo-de-Mora M, Quilendrino R, Ham L, Liarakos VS, van Dijk K, Baydoun L, Dapena I, Oellerich S, Melles GR (2015) Clinical outcome of 500 consecutive cases undergoing Descemet’s membrane endothelial keratoplasty. Ophthalmology 122(3):464–470. https://doi.org/10.1016/j.ophtha.2014.09.004
Birbal RS, Ni Dhubhghaill S, Bourgonje V, Hanko J, Ham L, Jager MJ, Böhringer S, Oellerich S, Melles G (2020) Five-Year graft survival and clinical outcomes of 500 consecutive cases after Descemet membrane endothelial keratoplasty. Cornea 39(3):290–297. https://doi.org/10.1097/ICO.0000000000002120
Stuart AJ, Romano V, Virgili G, Shortt AJ (2018) Descemet's membrane endothelial keratoplasty (DMEK) versus Descemet's stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst Rev 6(6), CD012097. https://doi.org/10.1002/14651858.CD012097.pub2
Woo JH, Ang M, Htoon HM, Tan D (2019) Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol 207:288–303. https://doi.org/10.1016/j.ajo.2019.06.012
Price DA, Kelley M, Price FW Jr, Price MO (2018) Five-year graft survival of Descemet membrane Endothelial Keratoplasty (EK) versus Descemet stripping EK and the effect of donor sex matching. Ophthalmology 125(10):1508–1514. https://doi.org/10.1016/j.ophtha.2018.03.050
Ham L, Dapena I, Liarakos VS, Baydoun L, van Dijk K, Ilyas A, Oellerich S, Melles GR (2016) Midterm results of Descemet membrane endothelial keratoplasty: 4 to 7 years clinical outcome. Am J Ophthalmol 171:113–121. https://doi.org/10.1016/j.ajo.2016.08.038
Vasiliauskaitė I, Oellerich S, Ham L, Dapena I, Baydoun L, van Dijk K, Melles G (2020) Descemet membrane endothelial keratoplasty: ten-year graft survival and clinical outcomes. Am J Ophthalmol 217:114–120. https://doi.org/10.1016/j.ajo.2020.04.005
Bourne WM (2001) Cellular changes in transplanted human corneas. Cornea 20(6):560–569. https://doi.org/10.1097/00003226-200108000-00002
Baydoun L, Ham L, Borderie V, Dapena I, Hou J, Frank LE, Oellerich S, Melles GR (2015) Endothelial survival after descemet membrane endothelial keratoplasty: effect of surgical indication and graft adherence status. JAMA ophthalmology 133(11):1277–1285. https://doi.org/10.1001/jamaophthalmol.2015.3064
Writing Committee for the Cornea Donor Study Research Group, Sugar A, Gal RL, Kollman C, Raghinaru D, Dontchev M, Croasdale CR, Feder RS, Holland EJ, Lass JH, Macy JI, Mannis MJ, Smith PW, Soukiasian SH, Beck RW (2015) Factors associated with corneal graft survival in the cornea donor study. JAMA Ophthalmol 133(3):246–254. https://doi.org/10.1001/jamaophthalmol.2014.3923
Tsao YT, Wu WC, Chen KJ, Yeh LK, Hwang YS, Hsueh YJ, Chen HC, Cheng CM (2020) Analysis of aqueous humor total antioxidant capacity and its correlation with corneal endothelial health. Bioeng Trans Med 6(2):e10199. https://doi.org/10.1002/btm2.10199
Yamaguchi T, Higa K, Tsubota K, Shimazaki J (2018) Elevation of preoperative recipient aqueous cytokine levels in eyes with primary graft failure after corneal transplantation. Mol Vis 24:613–620
Yazu H, Yamaguchi T, Aketa N, Higa K, Suzuki T, Yagi-Yaguchi Y, Satake Y, Abe T, Tsubota K, Shimazaki J (2018) Preoperative aqueous cytokine levels are associated with endothelial cell loss after Descemet’s stripping automated endothelial keratoplasty. Invest Ophthalmol Vis Sci 59(2):612–620
Kocaba V, Katikireddy KR, Gipson I, Price MO, Price FW, Jurkunas UV (2018) Association of the gutta-induced microenvironment with corneal endothelial cell behavior and demise in fuchs endothelial corneal dystrophy. JAMA ophthalmology 136(8):886–892
Schrittenlocher S, Schaub F, Hos D, Siebelmann S, Cursiefen C, Bachmann B (2018) Evolution of consecutive Descemet membrane endothelial keratoplasty outcomes throughout a 5-year period performed by two experienced surgeons. Am J Ophthalmol 190:171–178. https://doi.org/10.1016/j.ajo.2018.03.036
Satué M, Rodríguez-Calvo-de-Mora M, Naveiras M, Cabrerizo J, Dapena I, Melles GR (2015) Standardization of the Descemet membrane endothelial keratoplasty technique: outcomes of the first 450 consecutive cases. Archivos de la Sociedad Espanola de Oftalmologia 90(8):356–364. https://doi.org/10.1016/j.oftal.2015.01.004
Funding
Open Access funding enabled and organized by Projekt DEAL. No funds, grants, or other support were received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception of the work. The data were acquired by JW and TT. Data analysis and interpretation were performed by all authors. The manuscript was written by JW and revised critically by TT and FK. All authors approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Institutional Review Board (IRB)/ Ethics Committee of the Friedrich-Alexander University Erlangen-Nürnberg approved the study (Approval ID: 64_15 Bc).
Consent to participate
Informed consent to participate in the surgery and the retrospective study has been obtained from all study subjects. The authors state that no organs/tissues were obtained from prisoners. The corneal tissues were obtained from an eye bank in the United States (SightLife, Seattle), and from Europe (via German Society for Tissue Transplantation (DGFG).
Consent for publication
The authors affirm that the study participants provided informed consent for publication of the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weller, J.M., Kruse, F.E. & Tourtas, T. Descemet membrane endothelial keratoplasty: analysis of clinical outcomes of patients with 8–10 years follow-up. Int Ophthalmol 42, 1789–1798 (2022). https://doi.org/10.1007/s10792-021-02176-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-02176-3