Abstract
Introduction
The pandemic of COVID-19 has been caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. Apart from respiratory malfunction, COVID-19 causes a system-wide thromboembolic state, leading to serious cardiovascular, cerebrovascular and peripheral vascular manifestations. However, our knowledge regarding retinal manifestations due to systemic COVID-19 is minimal. This systematic review has comprehensively summarized all retinal manifestations secondary to COVID-19 disease recorded till date since the beginning of the pandemic.
Methods
All studies published till November 27, 2020, which have reported retinal manifestations in COVID-19 patients were systematically reviewed using the PRISMA statement.
Results
We included 15 articles: 11 case reports and four cross-sectional case series. The most commonly reported manifestations which did not affect visual acuity were retinal hemorrhages and cotton wool spots. The most common vision threatening manifestation was retinal vein occlusion with associated macular edema. Rarely, patients may also present with retinal arterial occlusions and ocular inflammation. These manifestations may occur from as soon as within a week after the onset of COVID-19 symptoms to more than 6 weeks after.
Conclusion
Mostly causing milder disease, COVID-19 may however lead to severe life-threatening thromboembolic complications, and systemic antithrombotic therapy has been suggested as a prophylactic and therapeutic management strategy for patients affected with serious systemic disease. However, both sick and apparently healthy patients may suffer from various retinal complications which may lead to loss of vision as well. No consensus regarding management of retinal complications with anticoagulants or anti-inflammatory medications have been proposed; however, they may be tackled on individual basis.
Similar content being viewed by others
Introductions
The coronavirus 2019 pandemic has spread worldwide claiming millions of lives, with various challenging clinical manifestations, prolonged recovery time and post-recovery complications. The disease begins with a mild clinical course with fever, dry cough, malaise, progressing most commonly to a mild-to-moderate lower respiratory tract disease, finally resolving without specific treatment [1]. Although the respiratory system is the most commonly affected organ, the virus shows neurotropism, endothelial tropism and may also cause a system-wide inflammatory reaction, known as the “cytokine storm.” A significant proportion of COVID-19 affected patients may show neurological and vascular manifestations [2, 3].
The proposed pathways by which the virus can gain entry into the central nervous system (CNS) are through blood stream into the cerebral circulation, where the slower blood flow might be conducive to the virus affecting the capillary endothelium, and dissemination through the olfactory bulb [4, 5]. Extensive study into the pathogenesis of vascular events has led researchers to conclude that COVID-19 may primarily be a vascular disease causing severe endothelial disruption, complement activation, and generalized inflammation, leading to an overall procoagulant state [6]. Along with the CNS, the neurosensory retina and the optic nerve also have been found to be affected in several reported cases since May 2020. The aim of this review was to create a systematic compilation of the retinal signs seen in COVID patients, as well as to understand and explore the mechanisms by which coronaviruses may affect the retina. Given that the COVID-19 pandemic is an evolving situation, the implications and inferences of this review may be early, although we would like to encourage the readers to deem the timeliness of this review relevant in clinical setups.
Methods
This study was performed according to the PRISMA guidelines and flow diagram for manuscript format development [7]. The review protocol was not previously registered.
Literature search
A systematic literature review was performed by two independent reviewers (for screening, eligibility and inclusion) from the electronic databases, PubMed and Google Scholar, for English language articles and English translated abstracts of non-English articles. To avoid missing any articles, the references of the relevant articles or reviews identified through search were also scanned. The literature search was performed on November 27, 2020, at 10 pm IST. The search strategy included terms “retina” and “COVID” in various combinations along with Boolean search operators “OR” and “AND.” The titles and abstracts were screened for all articles to ensure that they met eligibility criteria by the reviewers independently. Full texts of all titles which appeared to meet the inclusion criteria were downloaded. After screening full texts, the articles were finally included, and in case of any disagreements, they were solved by discussion.
Inclusion and exclusion criteria
We included any prospective and retrospective studies, along with case series, case reports and correspondence reporting retinal manifestations in confirmed COVID-19 patients, of any age, any gender and any race in this study. Patients were defined as confirmed COVID-19 positive cases either based on clinical criteria (recommended by their National Health agencies) or positive RT-PCR for viral RNA from nasopharyngeal swabs. Studies with only suspected cases of COVID-19 were excluded from analysis. Letter to editors not reporting cases, narrative reviews, and correspondences (such as editorials) were also excluded.
Data extraction
A Microsoft excel spreadsheet was used to extract data from the included eligible studies, related to study design and setting, demographic and clinical details of patients, reported ocular signs and symptoms, status of RT-PCR positivity, and systemic disease status. Two reviewers extracted the data independently and in case of any confusion or discrepancy, a third reviewer was consulted.
Study outcomes
The primary outcomes to be evaluated were presenting clinical features of patients, demographic profile, systemic profile, retinal complications of COVID-19 patients, severity and prognosis.
Results
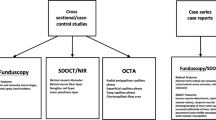
We identified 192 citations through the search of electronic databases. After removing duplicate articles, screening of titles and abstracts was performed for 171 articles. Full text screening was done for 35 manuscripts of which 15 fulfilled the inclusion criteria and were used for data extraction. The PRISMA flowchart shows the full screening process (Fig. 1). The characteristics of the included studies are listed in Table 1. A total of 121 confirmed cases of COVID-19 were reported in the included studies. Of the final eligible studies included for data extraction, there were 11 case reports and four cross-sectional case series.
Retinal hemorrhages and cotton wool spots
Pereira et al. [8] evaluated a series of 18 patients, with a median age of 62.5 years. A total of 95% of this series' patients were admitted in the ICU and nearly half were on vasopressor support; 55.5% patients had retinal findings. A total of 22.2% patients had flame hemorrhages, 16.7% had cotton wool spots, 11.1% had peripheral retinal hemorrhages, and 5.6% had macular hemorrhages and hard exudates. Flame hemorrhages signified an insult to the inner retina. One patient also showed sectoral retinal pallor, suggesting recent ischemic event.
In a larger series of 54 patients (SERPICO study), who were not critically sick, Invernizzi A et al. [9] found retinal hemorrhages in 9.25% patients and cotton wool spots in 7.4%. Inflammatory markers were raised in all the patients. Authors also noted that fibrinogen, CRP, ferritin and LDH levels were all raised in the patients.
D'Aloisio R et al. reported a case who developed bilateral retinal hemorrhages, involving the fovea in one patient [10]. These hemorrhages spontaneously resolved within a month. The retinal findings were detected because of blurred vision 28 days after the RT-PCR test came positive. At the final follow-up, the vision had recovered fully.
Marinho PM evaluated 11 COVID-19 positive patients, age range between 25 and 70, and observed that four patients had developed cotton wool spots and microhemorrhages along the retinal arcade [11].
Landecho et al. [12] studied 27 patients who were positive for the COVID-19 antibody and found cotton wool spots in 22% of patients, after a median period of 43 days from the first clinical symptoms of COVID-19. The D-dimer levels were raised in all patients with a median value of 385 at the time of funduscopic examination.
Venous occlusion
Central retinal vein occlusion (CRVO) has been reported in four patients till date [13,14,15,16]. The ages of the patients reported ranged from 17 to 54 years (median = 40 years). The time of presentation after the first onset of fever ranged from 5 days to 6 weeks. Associated macular edema was seen in 3/4 eyes, and the fourth patient had an impending CRVO. One patient was treated with sustained release dexamethasone implant (Ozurdex, Allergan, Dublin, Ireland), with a recovery of vision from 20/200 to 20/40 within a span of 2 weeks. The case reported by Invernizzi A et al. [13] had 20/40 visual acuity, retinal hemorrhages, increased venular tortuosity and a panretinal fern-like whitening (visible as perivenular hypoautofluorescence), which led the authors to diagnose the patient as having an impending CRVO. The patient responded to steroid therapy (initial intravenous followed by oral tapering dose). After a week, the patient had complete regression of the retinal changes. The case reported by Insausti-Garcia A presented as a papillophlebitis [14]. Sheth et al. [15] reported a case of inferior hemiretinal vein occlusion, with dilated and tortuous vessels which showed vessel wall staining, suggestive of a vasculitic etiology. Moreover, optical coherence tomography (OCT) was used to document disorganization of retinal inner layers along with the neurosensory detachment at fovea. The patient was treated with systemic steroids and ranibizumab for the macular edema, with an improvement of visual acuity from 20/200 to 20/30. Authors also noted that the D-dimer values of the patients were significantly raised. The 40-year-old patient who presented with CRVO within 5 days of COVID-related symptoms had associated deep vein thrombosis in the right leg, and heart echocardiography showed severe dilation of the right ventricle with raised right ventricle pressure and volume overload, consistent with features of right heart strain [16]. Moreover, this patient had bilateral CRVO, which is the only such case reported till date. IL-6 levels were also raised in the same patient. The patient was put on a therapeutic dose of low-molecular-weight heparin (LMWH). Walinjkar et al. [17] reported a 17-year-old COVID-19 patient with CRVO with macular edema, who was injected bevacizumab twice, and the visual acuity improved from 20/60 to 20/40 within 2 months.
Arterial occlusion
Acharya S et al. [18] reported a 60-year-old male patient who was admitted in ICU with acute respiratory distress syndrome and underwent mechanical ventilation secondary to COVID-19, with raised fibrinogen, D-Dimer levels and IL-6. After 12 days of hospital admission, patient developed cherry red spot in one eye with optic disk margin blurring and retinal whitening, suggestive of a central retinal arterial occlusion. Further clinical details of the patient have not been reported.
Gascon et al. [19] described a 53-year-old patient who developed an ill-defined intraretinal white lesion at fovea, with intraretinal hemorrhages, several extra macular deep retinal hemorrhages and Roth spots. OCT through the foveal lesion showed multiple hyperreflective bands involving the inner nuclear layer (INL) and outer plexiform layer (OPL), which was suggestive of paracentral acute middle maculopathy (PAMM). Ellipsoid and interdigitation zone attenuation with OPL and outer nuclear layer (ONL) hyperreflectivity, along with subretinal fluid were suggestive of acute macular neuroretinopathy (AMN). OCT angiography also showed reduced flow signal in the deep capillary plexus, corresponding to the areas of PAMM. Virgo et al. [20] described two post-COVID patients presenting with PAMM (35 days following febrile illness) and AMN (16 days following swab positivity). Both the patients had presented with new onset paracentral scotoma.
Vitritis
Zago Filho et al. [21] reported a 57-year-old woman who developed vitritis along with a yellowish macular lesion, 12 days after COVID-19 symptoms. OCT showed hyperreflective lesions at IPL and ganglion cell layer along with disruption of EZ, which persisted till 1 month, and reduced by 2 months in size and reflectivity. Probably, these lesions were AMN/ PAMM lesions with dropouts in the capillary plexus; however, the authors have not made a definitive conclusion, or done an angiography.
Posterior uveitis
Castanedo et al. [22] reported a case of an 11-year-old male patient who presented with edematous plaques in both lower limbs, which was diagnosed as chilblains. The child had a positive SARS-CoV-2 IgG antibody report. Ocular examination showed retinal vasculitis with perivascular infiltrate and retinal exudates in left eye, with no vitritis.
Pirraglia et al. [23] evaluated a set of 43 COVID-19 patients with severe lower respiratory tract infection requiring hospitalization, and found unilateral posterior chorioretinitis in one patient, which was attributed to a probable fungal etiology and was treated accordingly; however, the patient succumbed to COVID-19-related pneumonia after a month.
Vascular dilatation
In the SERPICO study, authors found that in COVID-19-exposed patients, both the vein and artery diameters were larger than in unexposed subjects [9]. The venular diameter was negatively correlated with the time interval between systemic onset of symptoms and the day of fundus photo capture, suggesting that this may be related to the level of inflammatory mediators released in the blood during early COVID-19 disease.
Discussion
Since the first outbreak of SARS-CoV-2 in China in 2019, the world has experienced a prolonged pandemic named as COVID-19 [24]. Although the majority of COVID-19 patients may experience mild-to-moderate symptoms, several high-risk-group patients may have severe disease requiring hospitalization and also intensive care [25]. These patients may suffer from acute respiratory distress syndrome and potentially succumb to the disease. The disease presents with a major insult to the immune system of the body, along with multiorgan failure [26].
Extensive investigations into the respiratory system and related causes of morbidity and mortality have given us knowledge regarding systemic pathogenesis of the disease [27, 28]. Severe COVID-19 pneumonia patients may also develop ocular signs and symptoms [29]. In the eye, acute conjunctivitis is the most common presentation [30]. Interestingly, some authors have observed that ocular manifestations may be the first sign of COVID-19 [31].
Virus localization in neurological structures and retina
Previously coronaviruses have been demonstrated to retrogradely travel along neurons through synapses by endocytosis or exocytosis into the brain [32, 33]. SARS-CoV and MERS viruses may directly induce neuronal death in the medullary respiratory center, by upregulation of inflammatory markers or by direct autophagy. A similar model for SARS-CoV-2 has been proposed, in which the virus infects a peripheral neuron and retrogradely travels to the brain by synaptic transport [34]. Recently, SARS-CoV-2 RNA has been detected in the postmortem retina of affected patients as well [35]. It has been hypothesized that the virus may cause retinal vasculitis and ischemia in the retina. However, since features like vascular dilation, retinal hemorrhages and cotton wool spots are non-specific symptoms, it is possible that they occur due to the systemic illness rather than a primary insult to the CNS by SARS-CoV-2.
Endothelial cell involvement
Recent evidence strongly suggests that COVID-19 is actually a vascular disease affecting the endothelium via the angiotensin-converting enzyme 2 (ACE2) receptor expressed in several body organs, including the retinal endothelial cells [36]. Endothelial cell involvement has been demonstrated in the lung, heart, kidney, intestine and brain. The virus can lead to endotheliitis and vasculitis in both the arterial and venous circulations, inducing cellular edema, congestion and immunothrombosis of smaller vessels, ultimately resulting in impaired circulation and organ ischemia [37, 38]. Under physiological conditions, endothelial cells maintain tonic vasodilation through production of nitric oxide [39]. When SARS-CoV-2 uses ACE2 to invade into the cell, the cell loses ACE2 activity leading to increased angiotensin II and reduced angiotensin conversion. Increased angiotensin II leads to vasoconstriction, thereby triggering thrombogenicity by increasing platelet and leucocyte adhesion [39]. Furthermore, products of vascular injury activate neutrophils via several signaling pathways, leading to more damage to the endothelial cell glycocalyx [40,41,42]. Pro-inflammatory factors released from the endothelial cells may result in a “cytokine storm,” causing further organ damage.
Venous thrombosis
Apart from the severe respiratory disease, the hallmark of COVID-19 disease are life-threatening multi-systemic thromboembolic complications which can lead to death even in asymptomatic individuals [43,44,45]. Postmortem evidence from COVID-19 patients have revealed severe endothelial injury with apoptosis, clotting, and multiple vessel thrombosis in most of the body organs [46, 47]. Apart from endotheliopathy, patients may also have endotheliitis. Most of these manifestations are due to the system-wide inflammatory reaction by the virus, with hypercoagulability, platelet activation and endothelial dysfunction [48].
Authors have recently observed that even after systematic use of thromboprophylaxis, around 30% of COVID-19 patients in ICU may develop thrombotic complications, and this may be associated with higher mortality [49,50,51,52,53]. The cumulative incidence of arterial and venous thromboembolism (VTE) has been estimated as 49% [54]. These studies suggest that along with anticoagulant therapy, treatment should be targeted toward endothelial injury as well. Moreover, as many as 17% non-ICU patients may also have VTE [55, 56].
Stroke has been reported as a rare complication of COVID-19 disease, and it may occur in patients with risk factors like hypertension, diabetes mellitus and cardiovascular comorbidities; these patients may have more severe systemic disease as well [57,58,59,60]. However, in the few cases without these risk factors, primary COVID-19-associated coagulopathy (CAC) may lead to cerebrovascular disease [61, 62]. In contrast to bacterial sepsis-induced disseminated intravascular coagulation (DIC), CAC shows minor changes to prothrombin time and platelet count, however presents with increased fibrinogen and D-Dimer levels [63, 64]. CAC may be associated with severe derangement of D-dimers in contract to DIC, and COVID-19 patients may score quite low on the “sepsis-induced coagulopathy score.” Hence, it has been suggested that CAC should actually be considered as an endotheliopathy rather than a coagulopathy [65, 66].
D-dimer levels during hospitalization has been significantly correlated with incident risk of VTE and mortality [56, 67, 68]. Hence, D-dimer level increase with elevated fibrinogen and von Willebrand factor with normal PR, aPTT and platelet count should signify a diagnosis of CAC, and patients can be monitored using D-dimer [69,70,71].
Arterial thrombosis
Authors have reported ischemic stroke and acute coronary syndrome in COVID-19 patients at incidence rates of 2.5% and 1.1%, respectively [44]. Previously, such arterial thrombi have been reported quite rarely in infectious diseases [72, 73]. Young patients have been especially noted to have large vessel stroke [43]. Other manifestations of arterial thromboembolism (ATE) like limb ischemia, common iliac artery thrombosis, and aortic thrombi have also been reported [74]. These events have occurred in the presence of elevated D-dimer levels along with a confirmed diagnosis of COVID-19, with no previous history of arterial disease. The exact mechanism of arterial thrombosis is still unknown, and may be related to the inflammatory cytokine storm, and D-dimer levels again may be a predictor of such events.
Antithrombotic prophylaxis
Routine antithrombotic prophylaxis may be advised in cases of critically ill patients with/ without elevated D-dimer levels in COVID-19 disease [54, 63, 75, 76]. Proper anticoagulation in adequate dose, in the form of subcutaneous low-molecular-weight heparin (LMWH) improves survival in hospital admitted patients [77]. Patients in whom diagnostic tests are not possible in an emergency state and who have less risk of bleeding should also be considered for therapeutic anticoagulation. Moreover, 11% of VTE events occurred after a median period of 8 days of post-discharge (1.6 times the rate from non-COVID era) [77]. However, extended prophylaxis may be associated with an increased risk of bleeding [78].
Post-discharge continuation of prophylactic anticoagulation is still a gray zone due to lack of data regarding incidence of thromboembolic events following discharge from hospital after a COVID-19 episode [79]. Currently, interim guidelines from the International Society on Thrombosis and Haemostasis (ISTH) and the American College of Cardiology (ACC) have recommended the use of prophylactic dose of LMWH or unfractionated heparin for any COVID-19 patient getting hospital admission, and in case of contraindications to anticoagulants, mechanical prophylaxis can be used. However, clinicians are also of the opinion that extended usage of anticoagulants needs to be advised after taking into consideration the ongoing thromboembolic risk factors, and the overall risk–benefit ratio [54, 80, 81]. At present, starting asymptomatic COVID-19 patients freshly on anticoagulants or antiplatelet medications solely due to occurrence of retinal vascular thromboembolic events is not warranted; however, a proper systemic evaluation may be necessary before any such treatment is initiated.
Vasculitis
Apart from endothelial damage by virus, endothelial cells may also undergo inflammation, apoptosis and dysfunction [82]. Italian clinicians have reported that during the COVID pandemic, an increasing number of patients presented with a Kawasaki-like disease with an acute vasculitis involving the coronary arteries [83]. Urticarial vasculitis has also been reported in COVID-19 patients [84]. Authors have observed inflammatory CNS vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in a patient of COVID-19, who showed clinical improvement after immunomodulation therapy [85]. Type 3 hypersensitivity and IL-6-mediated inflammation have been proposed as the pathophysiology of this COVID-19-induced vasculitis [86].
Pathomechanism in retina
Retinal vasculature may be affected by two mechanisms, hypercoagulability leading to a DIC-like state, and a vasculitis-like process, due to direct viral invasion of endothelial cells [37, 87]. In animal models, coronaviruses have been previously demonstrated to cause retinal vasculitis, retinal degeneration and breakdown of blood-retinal barrier [88]. Experimental coronavirus retinopathy (ECOR) model has shown that the virus-induced retinal damage happens in a biphasic manner. The early phase involves retinal inflammation and infiltration by immune cells and release of inflammatory mediators, and in the next phase, which comes after the first week of infection, viral clearance occurs. Later on, autoantibodies are produced against retina and retinal pigment epithelium cells, leading to damage of photoreceptors and neuroretina [89]. Moreover, coronaviruses may also lead to intraocular inflammation and vitritis [90].
Asymptomatic ocular microangiopathic syndrome has been observed commonly after other viral infections like the human immunodeficiency virus (HIV) disease or in systemic diseases like diabetes and hypertension [91, 92]. However, the manifestation of microangiopathy in such cases in the form of cotton wool spots may be different, for e.g., HIV-associated CWS may be more mid-peripheral than diabetic retinopathy (DR). The pathogenesis of the two types of CWS may be different, and the in case of viruses, may be due to a direct viral effect. It is unclear whether the retinal microangiopathy seen with SARS-CoV-2 is due to direct viral effect or similar to DR. SARS-CoV and SARS-CoV-2 viruses both lead to ACE2 downregulation and this has been proposed as the mechanism behind development of the retinal ischemia and endothelial disease [91, 93,94,95]. Moreover, apart from being a manifestation of disease itself, CWS may also be a marker for future vascular complications in COVID-19 patients, similar to diabetic and hypertensive vascular disease [96]. Also, signs of arterial microangiopathy on retina examination may help identify patients in whom antiplatelet therapy may be started in addition to anticoagulation.
Immunopathogenesis
Retinal degeneration in ECOR is associated with an increase in TNF-alpha levels and soluble TNFR-2, which leads to aberrant TNF signaling [97]. Although anti TNF-alpha agents may be considered for COVID-related manifestations, this may be risky in terms of triggering fulminant tuberculosis [98]. In such situation, steroids appear to play an important role in suppressing the severe inflammatory reaction [99, 100] Although the key inflammatory molecules which regulate the cytokine storm in COVID are as yet unclear, it has been noted that SARS-CoV-2 produces a cytokine storm composed of low level of type 1 cytokines and higher levels of type 2 cytokines; this type 2 cytokine activity may be further boosted by the secretion of IL-33, an alarmin belonging to the IL-1 family, from epithelial cells [101]. This may also be associated with neutrophil dysregulation. Trials for IL-33 blockade are currently going on (ClinicalTrials.gov Identifier NCT04386616) [101]. The conditions for the cytokine storm may also be affected by specific genetics and environmental factors [102]. The “threshold model” for SARS-COV-2 infection has compared the pathophysiology behind it to macrophage activation syndrome (MAS) [102]. Recent evidence has also indicated an involvement of inflammasome activation and pyroptosis pathway [103, 104]
Limitations
Most of the studies included in our systematic review are case reports and observational case series, and larger-scale studies with bigger number of cases and longer follow-ups are needed to truly understand the natural history of COVID-19-related retinal manifestations. We understand that the timing of the review may have a potential risk of premature analysis of disease presentations and their implications. However, our aim was to synthesize data so that current practicing physicians can update their knowledge regarding ophthalmic organ-related complications amidst all time constraints.
Conclusion
This systematic review has comprehensively summarized all retinal manifestations recorded till date secondary to COVID-19 disease since the beginning of the pandemic. Retinal findings may range from mild signs like hemorrhages and cotton wool spits, to more severe complications like retinal vein occlusions, arterial occlusions, localized retinal infarcts and ocular inflammation. These manifestations may occur as soon as within a week after the onset of COVID-19 symptoms to more than 6 weeks after. Clinicians must be aware of such complications in case of any clinical suspicion of symptoms or signs suggestive of ocular disease. Treatment targeted toward the ocular complications may help resolve the particular complication. There is however no consensus regarding systemic anticoagulant therapy solely for ocular complications, if the patient was not receiving any such therapy previously.
References
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet 395(10223):497–506
Nath A (2020) Neurologic complications of coronavirus infections. Neurology 94(19):809–810
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol 77(6):683–690
Baig AM, Khaleeq A, Ali U, Syeda H (2020) Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem Neurosci 11(7):995–998
Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S (2008) Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82(15):7264–75
Marchetti M (2020) COVID-19-driven endothelial damage: Complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann Hematol 99:1701–1707
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1-34
Pereira LA, Soares LCM, Nascimento PA, Cirillo LRN, Sakuma HT, Veiga GLD, et al. (2020) Retinal findings in hospitalised patients with severe COVID-19. Br J Ophthalmol, 16:bjophthalmol-2020–317576
Invernizzi A, Torre A, Parrulli S, Zicarelli F, Schiuma M, Colombo V et al (2020) Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine 27:100550
D’Aloisio R, Nasillo V, Gironi M, Mastropasqua R (2020) Bilateral macular hemorrhage in a patient with COVID-19. Am J Ophthalmol Case Rep. https://doi.org/10.1016/j.ajoc.2020.100958
Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R Jr (2020) Retinal findings in patients with COVID-19. Lancet 395(10237):1610
Landecho MF, Yuste JR, Gándara E, Sunsundegui P, Quiroga J, Alcaide AB et al (2020) COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease? J Intern Med 289(1):116–20
Invernizzi A, Pellegrini M, Messenio D, Cereda M, Olivieri P, Brambilla AM et al (2020) Impending Central Retinal Vein Occlusion in a Patient with Coronavirus Disease 2019 (COVID-19). Ocul Immunol Inflamm 28(8):1290–1292
Insausti-García A, Reche-Sainz JA, Ruiz-Arranz C, López Vázquez Á, Ferro-Osuna M (2020) Papillophlebitis in a COVID-19 patient: inflammation and hypercoagulable state. Eur J Ophthalmol 30:1120672120947591
Sheth JU, Narayanan R, Goyal J, Goyal V (2020) Retinal vein occlusion in COVID-19: a novel entity. Indian J Ophthalmol 68(10):2291–2293
Gaba WH, Ahmed D, Al Nuaimi RK, Dhanhani AA, Eatamadi H (2020) Bilateral central retinal vein occlusion in a 40-year-old man with severe coronavirus disease 2019 (COVID-19) Pneumonia. Am J Case Rep 21:e927691
Walinjkar JA, Makhija SC, Sharma HR, Morekar SR, Natarajan S (2020) Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian J Ophthalmol 68(11):2572–2574
Acharya S, Diamond M, Anwar S, Glaser A, Tyagi P (2020) Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases 21:e00867
Gascon P, Briantais A, Bertrand E, Ramtohul P, Comet A, Beylerian M et al (2020) Covid-19-associated retinopathy: a case report. Ocul Immunol Inflamm 28(8):1293–1297
Virgo J, Mohamed M (2020) Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Published online July, Eye
Zago Filho LA, Lima LH, Melo GB, Zett C, Farah ME (2020) Vitritis and outer retinal abnormalities in a patient with COVID-19. Ocul Immunol Inflamm 28(8):1298–1300
Quintana-Castanedo L, Feito-Rodríguez M, Fernández-Alcalde C, Granados-Fernández M, Montero-Vega D, Mayor-Ibarguren A et al (2020) Concurrent chilblains and retinal vasculitis in a child with COVID-19. J Eur Acad Dermatol Venereol. https://doi.org/10.1111/jdv.16801
Pirraglia MP, Ceccarelli G, Cerini A, Visioli G, d’Ettorre G, Mastroianni CM et al (2020) Retinal involvement and ocular findings in COVID-19 pneumonia patients. Sci Rep 10(1):17419
Jee Y (2020) WHO International Health Regulations Emergency Committee for the COVID-19 outbreak. Epidemiol Health 42:e2020013
Grasselli G, Pesenti A, Cecconi M (2020) Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 323(16):1545–1546
Sarzi-Puttini P, Giorgi V, Sirotti S et al (2020) COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol 38(2):337–342
Long B, Brady WJ, Koyfman A, Gottlieb M (2020) Cardiovascular complications in COVID-19. Am J Emerg Med 38(7):1504–1507
Baig AM (2020) Neurological manifestations in COVID-19 caused by SARS-CoV-2. Ther CNS Neurosci 26(5):499–501
Wu P, Duan F, Luo C (2020) Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei province. China JAMA Ophthalmol 138(5):575–578
Cheema M, Aghazadeh H, Nazarali S et al (2020) Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Can J Ophthalmol. https://doi.org/10.1016/j.jcjo.2020.03.003
Daruich A, Martin D, Bremond-Gignac D (2020) Ocular manifestation as first sign of coronavirus disease 2019 (COVID-19): interest of telemedicine during the pandemic context. J Fr Ophtalmol 43:389–391
Dubé M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ (2018) (2018) Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol 92(17):1–21
Munster VJ, Prescott JB, Bushmaker T, Long D, Rosenke R, Thomas T et al (2012) Rapid Nipah virus entry into the central nervous system of hamsters via the olfactory route. Sci Rep 2:1–8
Baig AM et al (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms, ACS Chem. Neurosci 11:995–998
Casagrande M, Fitzek A, Pu¨schel K et al (2020) Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm 28:721–725
Senanayake P, Drazba J, Shadrach K et al (2007) Angiotensin II and its receptor subtypes in the human retina. Invest Ophthalmol Vis Sci 48(7):3301–3311
Varga Z, Flammer AJ, Steiger P et al (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395:1417–1418
Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A et al (2020) Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 6:M20-2003
Verdecchia P, Cavallini C, Spanevello A, Angeli F (2020) COVID-19: ACE2 centric infective disease? Hypertension 76(2):294–299
Yamaguchi Y, Moriki T, Igari A, Matsubara Y, Ohnishi T, Hosokawa K, Murata M (2013) Studies of a microchip flowchamber system to characterize whole blood thrombogenicity in healthy individuals. Thromb Res 132:263–270
Leavy O (2015) NETting a one-two punch. Nat Rev Immunol 15:526–527
Hirota T, Levy JH (1994) Iba T (2020) The influence of hyperglycaemia on neutrophil extracellular trap formation and endothelial glycocalyx damage in a mouse model of type 2 diabetes. Microcirculation (New York, NY
Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP et al (2020) Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 382(20):e60
Lodigiani C, Iapichino G, Carenzo L et al (2020) Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 191:9–14
Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E et al (2020) Global COVID-19 thrombosis collaborative group, endorsed by the ISTH, NATF, ESVM, and the IUA, supported by the ESC working group on pulmonary circulation and right ventricular function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 75(23):2950–2973
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F et al (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383(2):120–128
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H (2020) Endothelial cell infection and endotheliitis in COVID- 19. Lancet 395:1417–1418
Levi M, Thachil J, Iba T, Levy JH (2020) Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 7(6):e438–e440440
Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res S0049–3848(20):30120–1
Klok FA, Kruip MJHA, van der Meer NJM (2020) Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 191:148–150
Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K et al (2020) Cerebral venous thrombosis associated with COVID-19. Am J Neuroradiol 41:1370–1376
Porfidia A, Pola R (2020) Venous thromboembolism in COVID-19 patients. J Thromb Haemost 18(6):1516–1517
Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, Zhang J, Zhao C (2020) Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol 92(10):1902–14
Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M et al (2020) ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 18(5):1023–1026
Marchandot B, Sattler L, Jesel L, Matsushita K, Schini-Kerth V, Grunebaum L et al (2020) COVID-19 Related coagulopathy: a distinct entity? J Clin Med 9(6):1651
Artifoni M, Danic G, Gautier G, Gicquel P, Boutoille D, Raffi F et al (2020) Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis 50(1):211–216
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q et al (2020) Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan. China. JAMA Neurol 77(6):683–690
Guo J, Huang Z, Lin L, Lv J (2020) Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc 9(7):e016219
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q et al (2020) Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 94:91–95
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062
Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S et al (2020) COVID-19 presenting as stroke. Brain Behav Immun 87:115–119
Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V et al (2020) Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 18(7):1738–1742
Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020 4;135(23):2033–2040. https://doi.org/10.1182/blood.2020006000
Iba T, Levy JH (2020) Sepsis-induced coagulopathy and disseminated intravascular coagulation. Anesthesiology 132(5):1238–1245
Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. https://doi.org/10.1007/s00134-020-06062-x
Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P et al (2020) Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 7(8):e575–e582
Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y et al (2020) Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol 5(3):279–284
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054
Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M et al (2020) The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 18(7):1747–1751
Oudkerk M, Büller HR, Kuijpers D, van Es N, Oudkerk SF, McLoud T et al (2020) Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the national institute for public health of the Netherlands. Radiology 297:E216–E222
Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W et al (2020) Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 382:e38
Escher R, Breakey N, Lämmle B (2020) Severe COVID-19 infection associated with endothelial activation. Thromb Res 190:62
Bunce PE, High SM, Nadjafi M, Stanley K, Liles WC, Christian MD (2016) Pandemic H1N1 influenza infection and vascular thrombosis. Clin Infect Dis 52:e14–e17
Garg K, Barfield ME, Pezold ML, Sadek M, Cayne NS, Lugo J et al (2020) Arterial thromboembolism associated with COVID-19 and elevated D-dimer levels. J Vasc Surg Cases Innov Tech 6(3):348–351
Ren B, Yan F, Deng Z, Zhang S, Xiao L, Wu M, Cai L (2020) Extremely High Incidence of Lower Extremity Deep Venous Thrombosis in 48 Patients With Severe COVID-19 in Wuhan. Circulation 142(2):181–183
Paranjpe I, Fuster V, Lala A (2020) Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. https://doi.org/10.1016/j.jacc.2020.05.01
Roberts LN, Whyte MB, Georgiou L, Giron G, Czuprynska J, Rea C et al (2020) Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood 136(11):1347–1350
Cohen AT, Harrington RA, Goldhaber SZ, Hull RD, Wiens BL, Gold A, Investigators APEX et al (2016) Extended Thromboprophylaxis with Betrixaban in Acutely Ill Medical Patients. N Engl J Med 375(6):534–44
Tao DL, Bien JY, DeLoughery TG, Shatzel JJ (2017) Extended thromboprophylaxis with direct oral anticoagulants for medical patients: a systematic review and meta-analysis. Blood 129(5):653–655
Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E et al (2020) J Am Coll Cardiol 75(23):2950–2973
Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A et al (2020) Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis 50(1):72–81
Becker RC (2020) COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis 50:54–67
Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D’Antiga L (2020) An outbreak of severe Kawasaki- like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395(10239):1771–1778
de Perosanz-Lobo D, Fernandez-Nieto D, Burgos-Blasco P, Selda-Enriquez G, Carretero I, Moreno C et al (2020) Urticarial vasculitis in COVID-19 infection: a vasculopathyrelated symptom? J Eur Acad Dermatol Venereol JEADV. https://doi.org/10.1111/jdv.16713
Pinto AA, Carroll LS, Nar V, Varatharaj A, Galea I (2020) CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurol Neuroimmunol Neuroinflamm 7:e813
Roncati L, Ligabue G, Fabbiani L, Malagoli C, Gallo G, Lusenti B et al (2020) Type 3 hypersensitivity in COVID-19 vasculitis. Clin Immunol 29:108487
Tang N, Li D, Wang X, Sun Z (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18:844–847
Seah I, Agrawal R (2020) Can the coronavirus disease 2019 (COVID-19) affect the eyes? a review of coronaviruses and ocular implications in humans and animals. Ocul immunol Inflamm 28:391–395
Hooks JJ, Percopo C, Wang Y, Detrick B (1993) Retina and retinal pigment epithelial cell autoantibodies are produced during murine coronavirus retinopathy. J Immunol 151(6):3381–9
Doherty MJ (1971) Ocular manifestations of feline infectious peritonitis. J Am Vet Med Assoc 159(4):417–24
Akram MU, Akbar S, Hassan T, Khawaja SG, Yasin U, Basit I (2020) Data on fundus images for vessels segmentation, detection of hypertensive retinopathy, diabetic retinopathy and papilledema. Data Brief 29:105282
Jaworski C (2000) Morphology of the HIV versus the diabetic cotton wool spot. Optom Vis Sci 77:600–604
Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46:586–590
Verma A, Shan Z, Lei B et al (2012) ACE2 and Ang-(1–7) confer protection against development of diabetic retinopathy. Mol Ther 20:28–36
Duan Y, Beli E, Li Calzi S et al (2018) Loss of angiotensin-converting enzyme 2 exacerbates diabetic retinopathy by promoting bone marrow dysfunction. Stem Cells 36:1430–1440
Mahdjoubi A, Bousnina Y, Barrande G, Bensmaine F, Chahed S, Ghezzaz A (2020) Features of cotton wool spots in diabetic retinopathy: a spectral-domain optical coherence tomography angiography study. Int Ophthalmol 40:1625–1640
Hooper LC, Chin MS, Detrick B, Hooks JJ (2005) Retinal degeneration in experimental coronavirus retinopathy (ECOR) is associated with increased TNF-alpha, soluble TNFR2 and altered TNF-alpha signaling. J Neuroimmunol 166(1–2):65–74
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) HLH across speciality collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395(10229):1033–1034
Xu J, Tan D, Fu Y, Walline J, Yu X (2015) Do corticosteroids have a role in treating Ebola virus disease? Sci China Life Sci 58(1):111–113
Neri P, Pichi F (2020) COVID-19 and the eye immunity: lesson learned from the past and possible new therapeutic insights. Int Ophthalmol 40(5):1057–1060. https://doi.org/10.1007/s10792-020-01389-2
Liang Y, Ge Y, Sun J (2021) IL-33 in COVID-19: a friend or foe? Cell Mol Immunol 18:1602–1604
Schulert GS, Cron RQ (2020) The genetics of macrophage activation syndrome. Genes Immun. https://doi.org/10.1038/s41435-020-0098-4
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20(6):363–374
Neri P, Lamperti M, Pichi F (2020) SARS-COV-2 and eye immunity: the lesson was learned but we are not done yet. Brainstorming on possible pathophysiology inspired by ocular models. Int Ophthalmol 40(8):1879–1883. https://doi.org/10.1007/s10792-020-01495-1
Funding
No funding was received for this manuscript.
Author information
Authors and Affiliations
Contributions
SS involved in literature review, data synthesis, writing, and proofing; NBK involved in writing and proofing; JK involved in literature review, data synthesis, writing, and proofing; RPR involved in writing and proofing; KK involved in writing and proofing; GB involved in writing and proofing; HR involved in writing and proofing; AU involved in writing and proofing; KR involved in writing and proofing.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sen, S., Kannan, N.B., Kumar, J. et al. Retinal manifestations in patients with SARS-CoV-2 infection and pathogenetic implications: a systematic review. Int Ophthalmol 42, 323–336 (2022). https://doi.org/10.1007/s10792-021-01996-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01996-7