Abstract
Purpose
To investigate the clinical efficacy of 0.01% atropine in slowing the progression of myopia in children and to evaluate the influence of 0.01% atropine on secretion of basal tear and stability of tear film.
Methods
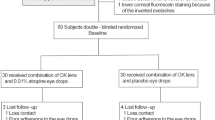
Eighty children aged 5–14 years with myopia, 40 were randomly divided into two groups consisting of those who received spectacles in addition to 0.01% atropine (SA group) and those who received only spectacles (S group). The remaining 40 children who were wearing orthokeratology (OK) lenses for 3 months were randomly divided into two groups comprising those who received OK lenses in addition to 0.01% atropine (OKA group) and those who received only OK lenses (OK group). Comprehensive ophthalmologic examinations, including slit-lamp examination, visual acuity testing, autorefraction, intraocular pressure, axial length (AL), corneal topography, Schirmer’s test, and tear film break-up time (TBuT), were performed before treatment and after every 3 months treatment.
Results
During the follow-up visits, evidently better spherical equivalent (SE) control over 3, 6 and 12 months was observed in the SA and OKA groups compared with the S and OK groups. The AL over 3, 6, and 12 months was evidently inhibited in the SA and OKA groups compared with the S and OK groups. No statistically significant differences in Schirmer’s test and TBuT results were observed between the S and SA groups and between the OK and OKA groups. However, statistically significant differences were found in TBuT results between before treatment and after 3 months treatment in the OK group (P < 0.05, paired t test) and the OKA group (P < 0.05, paired t test).
Conclusions
0.01% atropine can effectively control myopia progression and axial elongation regardless of combined treatment with spectacles or OK lenses. And 0.01% atropine has no evident effect on Schirmer’s test and TBuT results; however, researchers also found that Schirmer’s test and TBuT results showed a tendency to reduce after treatment with 0.01% atropine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myopia is a refractive error in which the refractive power is too large relative to the axial length of the eye. That is to say, in the static state of adjustment, external parallel light enters the eye and focuses before the photoreceptor layer of the retina, resulting in blurred far vision and clear near vision. Once myopia is formed, it will be irreversible. Nowadays, myopia is a common refractive error in children [1]. The prevalence of pediatric myopia reached to very high levels worldwide, especially in East Asian countries such as China [2]. Researchers predicted that approximately 50% of the population will be myopic by 2050, almost 1 billion people will exhibit high myopia [3]. As myopia tends to progress more rapidly when it occurs in a younger age and myopia progresses until adulthood [4, 5], controlling the development of myopia in adolescents is an urgent problem. A high myopia of ≥ 6 D increases the risk for vitreous opacity, macular degeneration, posterior sclera staphyloma, choroidal neovascularization, which is closely related to cataract, glaucoma, retinal detachment, and even blindness [2]. Multiple interventional methods, including pharmaceutical and optical treatments, were proposed to slow down myopia progression in children [6]. The muscarinic receptor antagonist 0.01% atropine was considered to be the most effective and safe treatment [6, 7]. However, we still require much experimental data to evaluate the clinical efficacy of 0.01% atropine in controlling myopia and providing guidance for clinical practice. In addition, concerns exist regarding whether accumulation of drug toxicity occurs and whether long-term use of 0.01% atropine would cause dry eyes. Therefore, analyzing the influence of 0.01% atropine on tear secretion and stability of tear film is also necessary.
Materials and methods
Patients
We included 80 children of ages 5–14 years with myopia at the Second Affiliated Hospital of Dalian Medical University during the years January 2019–April 2020. Before the experiment, the investigators explained the expected benefits and potential risks of using 0.01% atropine and OK lenses to the parents of children and also informed the detailed methods and precautions. This study was reviewed and approved by the Institutional Review Board of the Second Affiliated Hospital of Dalian Medical University (Dalian, China) and adhered to the tenets of the Declaration of Helsinki. In addition, consent was obtained from the parents of patients. Explanation was provided to children using an easy-to-understand method before obtaining informed consent.
Inclusion and exclusion criteria
The inclusion criteria were as follows: cycloplegic spherical equivalent refraction (SE) at least − 1.00 diopters (D) and diopter of spherical within − 1.00 to − 6.00 DS and myopic astigmatism ≤ − 1.00 DC and less than or equal to half the spherical diopter.
The exclusion criteria were as follows: (1) wearing contact lenses within 3 days at the start of examination, (2) children with ocular disorders such as glaucoma, cataract, keratopathy, strabismus, and amblyopia, and systemic disorders such as cardiac and respiratory illnesses, (3) intraocular pressure > 21 mm Hg and difference between the eyes > 8 mm Hg, (4) use of anticholinergic and cholinergic drugs that affect the evaluation of efficacy, such as atropine, pirenzepine, and pilocarpine within the past 1 month, (5) use of other therapies that may affect the evaluation of efficacy within the past 3 months, such as wearing OK lenses and therapy of traditional Chinese medicine, (6) low birth weight (≤ 1500 g), and (7) history of hypersensitivity to atropine or anticholinergic drugs.
Ophthalmic examinations
We applied 1% atropine eye gel for cycloplegic autorefraction after 3 days in children aged ≤ 10 years. The compound tropicamide was applied three times with an interval of 10 min to children aged > 10 years, and we waited for 20 min for cycloplegic autorefraction.
Subjects were prescribed 0.01% atropine eye drops to be applied once per night before bedtime in both eyes in both the SA and OKA groups. Subjects were required to wear OK lenses for at least 8 h every night in the OKA and OK groups. Additionally, OK lenses were worn 10 min after the use of 0.01% atropine in the OKA group. Spectacles were rematched when the SE dropped by 0.5 D and the vision of wearing glasses decreased. OK lenses were rematched when the naked vision during the day reached < 0.5. Further, 0.01% atropine is produced by Shenyang Xingqi pharmaceutical company (Shenyang, China). There were no preservatives and the PH value between 3.5 and 4.0 in 0.01% atropine.
When the central corneal thickness stabilized after the first 1–2 months of OK treatment, the axial length measurement was used as the baseline value after 3 months as in the studies conducted by Kakita et al [8]. The patients ceased wearing OK lenses and were examined weekly until the corneal curvature regressed and the refractive error stabilized (difference between consecutive visits ≤ 0.25 D) during the follow-up visit [8]. In this study, SE was measured using the TOPCON (KR-800). IOP was measured using the TOPCON (CT-IP). Corneal topography was evaluated using the OPD-Scan III. AL was measured using the Biometer (LS 900). All experimental data were recorded using the average of five successive measurements for analysis.
Statistical analysis
We applied SPSS 26.0 (IBM Corp., Armonk, NY) statistical analysis software for data analysis. The results of age, SE, AL, Schirmer’s test and TBuT results were described by mean (SD, standard deviation). Independent samples t tests were used for intergroup comparisons, and paired samples t test was used for intragroup comparisons. The Chi-square test was used to analyze gender differences between groups. P < 0.05 was considered statistically significant.
Results
A total of 160 eyes from 80 subjects were included in this study. No statistically significant differences were found between the S and SA groups and between the OK and OKA groups in terms of age, gender, SE, AL, Schirmer’s test, or TBuT results when they started treatment (Table 1).
The increases in SE were − 0.33 ± 0.14, − 0.67 ± 0.17, and − 1.30 ± 0.44 D, respectively, in the S group and − 0.11 ± 0.07, − 0.19 ± 0.08, and − 0.34 ± 0.16 D, respectively, in the SA group (P < 0.05, independent samples t test) over 3, 6, and 12 months. The increases in SE were − 0.14 ± 0.09, − 0.24 ± 0.15, and − 0.33 ± 0.16 D, respectively, in the OK group and − 0.05 ± 0.06, − 0.10 ± 0.08, and − 0.15 ± 0.08 D, respectively, in the OKA group (P < 0.05, independent samples t-test) over 3, 6, and 12 months (Table 1).
Regarding the increases in AL, the values were 0.13 ± 0.05, 0.31 ± 0.06, and 0.72 ± 0.21 mm, respectively, in the S group and 0.03 ± 0.01, 0.10 ± 0.04, and 0.24 ± 0.12 mm, respectively, in the SA group (P < 0.05, independent samples t test) over 3, 6, and 12 months. In the OK group, the increases in AL were 0.07 ± 0.03, 0.19 ± 0.13, and 0.29 ± 0.11 mm, respectively, and those in the OKA group were 0.02 ± 0.02, 0.08 ± 0.05, and 0.14 ± 0.08 mm, respectively (P < 0.05, independent samples t test) over 3, 6, and 12 months (Table 1).
No statistically significant differences were found in Schirmer’s test and TBuT results between the S and SA groups and also between the OK and OKA groups. However, statistically significant differences were found in TBuT results between before treatment and after treatment in the OK and OKA groups (both P < 0.05, paired samples t test), especially in the first 3 months; however, it almost recovered to pretreatment levels after 1 year. The Schirmer’s test and TBuT results showed a tendency to reduce in the SA and OKA groups even when all patients exhibited no symptoms and the data showed no statistical significance (Tables 2 and 3).
Discussion
With the increase in academic stress and the decrease in time spent in outdoor activities, the problem of myopia is increasing in terms of both incidence and severity in children. In addition, children-onset myopia is associated with the development of high myopia, which could result in several pathological complications such as cataracts, glaucoma, macular degeneration, retinal detachment, and even blindness [2]. Myopia has been considered as the sixth most common blindness cause, after ocular diseases such as cataracts, glaucoma, diabetic retinopathy and macular degeneration [9]. Therefore, we must take action to slow the development of myopia.
Juvenile myopia progression is mediated by both genetic and environmental factors. The cause and mechanism of the onset and progression of myopia are still not well understood. To explain the increase in the onset of myopia in school-aged children, several theories were proposed, including fewer outdoor activities, more near distance work, bad reading and writing habits, lighting, nutrition, and sleep [10, 11]. Meanwhile, several studies demonstrated that the risk of developing myopia in adolescents is significantly related to their parents because children with highly myopic parents were found to exhibit a rapid myopia progression rate [12].
Atropine and OK lenses are the commonly used methods to control myopia. Atropine is a nonselective mAChR antagonist; however, how atropine reduces the progression of myopia is unclear, although several possible mechanisms exist. A previous animal study demonstrated that atropine appears to block the M1 and M4 receptors in the retina and sclera that restrain axial elongation and reduce myopia progression by affecting the remodeling of sclera and reducing vitreous chamber growth [13]. Atropine also indirectly acts on the retina, causing retinal pigment epithelial cells or melanocytes to produce new chemicals (such as dopamine) and then acts on the sclera, which in turn inhibits thinning or stretching of the sclera to retard myopia progression [14]. Furthermore, atropine increases ultraviolet exposure in some extent due to mydriasis after using atropine may increase collagen cross-linking within the sclera, thereby limiting scleral extension [15]. Myopia may also be associated with increased ocular chronic inflammation, which may be reduced by atropine [16]. Another commonly accepted mechanism of atropine action is that it relieves nearwork-induced transient myopia (NITM). NITM is a transient myopia drift of near work for a period of time and a crucial factor related to the development of permanent myopia (PM) [17]. Currently, NITM is a complex problem that has not been well understood. Recent study has shown that 0.01% atropine have no clinical effect on corneal and lens; therefore it has almost no side effect. The effects of atropine mainly act on reducing AL elongation [18]. One study [19] reported that adverse reactions were not significantly related to 0.01% atropine.
OK is another important optical method used to control myopia progression, which is a technique that uses reverse-geometry-designed hard oxygen-permeable contact lens that is worn while sleeping to reshape the cornea momentarily to correct refractive error and improve naked vision during the day [20]. Currently, several studies show the efficacy of OK lenses in slowing the progression of myopia and axial elongation in children [21, 22]; however, the results vary greatly among individuals.
In this study, an evidently better SE control over 12 months was observed in the SA and OKA groups compared with the S and OK groups. The AL over 12 months was evidently restrained in the SA and OKA groups compared to that in the S and OK groups. Hence, 0.01% atropine can more effectively control myopia progression and axial elongation even when combined with spectacles or OK lenses.
The TBuT results were reduced after treatment with OK lenses irrespective of receiving 0.01% atropine. This is because OK lenses may influence the quality and stability of tear film. The mechanism underlying dry eye symptoms caused by wearing OK lenses remains unclear; however, several clinical studies confirmed that wearing corneal contact lenses for a long time will exhibit an effect on the amount and quality of tear secretion and dynamic distribution of tear film [23, 24]. The direct contact between OK lenses and tear film at night exhibits an effect on the physiological environment of the surface epithelium and tear, which destroys the connection between tear film and microvilli of epithelial cells, resulting in normal secretion of tear but difficult to form a stable tear film [25]. In this study, the TBuT results decreased more obviously in the first 3 months and then increased after 3 months, and it almost recovered to pretreatment levels after 1 year. All the enrolled patients exhibited no dry eye symptoms, and the experimental data showed no statistical significance during the follow-up; however, the Schirmer’s test and TBuT results showed a tendency to slightly reduce after treatment with 0.01% atropine. The possible reason is the inhibitory effect of atropine on the secretion of glands. However, the specific mechanism and the long-term effect of atropine on lacrimal secretion need to be explored.
In the current clinical treatment, questions still remain about which children would best benefit from treatment (e.g., in terms of age, level of myopia, rate of progression, and family risk factors), when atropine should be started and stopped, and for how long it should be used. Furthermore, we did not take into consideration the environmental factors such as near work time and outdoor activity time and the pupil diameters of the patients during the follow-up visits. In this study, the number of children was limited and the follow-up time was only 1 year. Therefore, further long-term follow-up studies are needed. We intend to continue to enroll additional subjects and conduct follow-up for a longer period of time.
Conclusion
This study provides clinical evidence indicating that 0.01% atropine can effectively control myopia progression and axial elongation regardless of combined treatment with spectacles or OK lenses. The TBuT reduced after treatment with OK lenses, especially in the first 3 months irrespective of receiving 0.01% atropine. However, it almost recovered to the pretreatment levels after 1 year. Researchers also found that Schirmer and TBuT results showed a tendency to reduce after treatment with 0.01% atropine, even when all patients exhibited no symptoms and data not showing statistical significance.
Data availability
All data and material are available from supplementary material.
References
Zadnik K, Sinnott LT, Cotter SA et al (2015) Prediction of juvenile-onset myopia. JAMA Ophthalmol 133:683–689
Morgan IG, Ohno-Matsui K, Saw SM (2012) Myopia. Lance 379(9827):1739–1748
Holden BA, Fricke TR, Wilson DA et al (2016) Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123(5):1036–1042
Grzybowski A, Kanclerz P, Tsubota K et al (2020) A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol 20(3):1–11
Chua SY, Sabanayagam C, Cheung YB, Chia A, Valenzuela RK, Tan D et al (2016) Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt 36:388–394
Huang J, Wen D, Wang Q, McAlinden C, Flitcroft I, Chen H et al (2016) Efficacy comparison of 16 interventions for myopia control in children: a network metaanalysis. Ophthalmology 123:697–708
Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A et al (2012) Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 119:347–354
Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T (2012) Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci 53:3913–3919
Morgan IG, He M, Rose KA (2017) Epidemic of pathologic myopia: what can laboratory studies and epidemiology tell us? Retina 37:989–997
Ip JM, Saw SM, Rose KA et al (2008) Role of near work in myopia: findings in a sample of Australian school children. Invest Ophthalmol Vis Sci 49:2903–2910
Rose KA, Morgan IG, Ip J et al (2008) Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 115:1279–1285
Xiang F, He M, Morgan IG (2012) The impact of parental myopia on myopia in Chinese children:population-based evidence J. Optom Vis Sci 89(10):1487–1496
Gallego P, Martinez-Garcia C, Perez-Merino P, Ibares-Frias L, Mayo-Iscar A, Merayo-Lloves J (2012) Scleral changes induced by atropine in chicks as an experimental model of myopia. Ophthalmic Physiol Opt 32:478–484
Metlapally R, Wildsoet CF (2015) Scleral mechanisms underlying ocular growth and myopia in progress. In molecular biology and translational science. Elsevier, Amsterdam
Prepas SB (2008) Light, literacy and the absence of ultraviolet radiation in the development of myopia. Med Hypotheses 70:635–637
Lin HJ, Wei CC, Chang CY et al (2016) Role of chronic inflammation in myopia progression: clinical evidence and experimental validation. EBioMedicine 10:269–281
Lin Z, Vasudevan B, Liang YB et al (2013) Near work induced transient myopia (NITM) in anisometropia. Ophthalmic Physiol Opt 33:311–317
Li FF, Kam KW, Zhang Y, Effects on Ocular Biometrics by 0.05%, 0.025%, and 0.01% Atropine: Low-concentration Atropine for Myopia Progression (LAMP) Study. Ophthalmology. 2020; 10.1016.
Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, Ko ST, Young AL, Tham CC, Chen LJ, Pang CP (2019) Low-concentration atropine for myopia progression (LAMP) study: a randomized, doubleblinded, placebocontrolled trial of 0.05%, and 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 126(1):113–124
Nichols JJ, Marsich MM, Nguyen M, Barr JT, Bullimore MA (2000) Overnight orthokeratology. Optom Vis Sci 77:252–259
Santodomingo-Rubido J, Villa-Collar C, Gilmartin B et al (2017) Long-term efficacy of orthokeratology contact lens wear in controlling the progression of childhood myopia. Curr Eye Res 42:713–720
Cho P, Tan Q (2019) Myopia and orthokeratology for myopia control. ClinExpOptom 102:364–377
Kastelan S, LukendaSalopek-rabati AA, Pavan J, Gotovac M (2013) Dry eye symptoms and signs in long-term contact lens wearers. J CollAntropol 37(1):199203
Szczesnaiskander DH, Iskander DR (2014) Tear film dynamics on soft contact lenses[J]. Optom Vis Sci 91(12):14061411
Zhao KX, Yang PZ (2013) Ophthalmology[M]. People’s Medical Press, Beijing, p 9294
Funding
This study was funded by Life Science Society of Liaoning.
Author information
Authors and Affiliations
Contributions
QH participated in the design of the study, carried out the study and drafted the manuscript and performed the statistical analyses. QZ has participated in the study’s coordination and has helped to draft the manuscript and has been involved in revising the manuscript carefully. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the second hospital of Dalian Medical University and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consents were obtained from the subjects after explanation of the nature of the study.
Consent to publish
The parents of patients agreed to collect and publish the data of their children.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Q., Hao, Q. Clinical efficacy of 0.01% atropine in retarding the progression of myopia in children. Int Ophthalmol 41, 1011–1017 (2021). https://doi.org/10.1007/s10792-020-01658-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01658-0